Translate this page into:
Erosive adenomatosis of the nipple in a man
2 Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, China
Correspondence Address:
Hong Jia
Department of Dermatology, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, 12, Jiangwangmiao Street, Nanjing, Jiangsu Province, 210042
China
| How to cite this article: Gu X, Wang G, Wu R, Jia H. Erosive adenomatosis of the nipple in a man. Indian J Dermatol Venereol Leprol 2015;81:68-70 |
Sir,
A 49-year-old Chinese man presented to our department with a 4-year history of erosions over the right nipple. The lesion had started as an area of erythema which gradually extended to the entire nipple. There was superficial ulceration on the nipple with crusting and serous discharge. There was aggravation with appearance of burning sensation on topical application of compound dexamethasone acetate. There was no family history of breast disease. Physical examination revealed an irregular ulcer on right nipple with slight serous exudate [Figure - 1]. On examination, no breast lump or enlarged lymph nodes were detected. Reports of ultrasound scanning and bilateral mammography were negative.
 |
| Figure 1: Swelling over right nipple, with an irregular ulcer and slight serous exudate |
Histopathological examination revealed a tubular mass in the dermis. The tube was lined with cuboidal myoepithelial cells on the outside and several layers of columnar epithelium within. Inner papillary proliferation and decapitation secretion into the lumen was seen. The stroma was proliferative and infiltrated with lymphocytes and plasma cells. There were no mitotic figures seen [Figure - 2]. On immunohistochemistry [Figure - 3], the inner cells were positive to cytokeratin 7 (CK7) and S-100, but negative to epithelial membrane antigen (EMA) and estrogen receptor (ER). The outer myoepithelial layer was positive to p63 and negative to p53. Ki67 staining showed positivity in 10% of cells. A diagnosis of erosive adenomatosis of the nipple (EAN) was made. The nipple was totally excised, and no relapse was noted after 1 year of follow up.
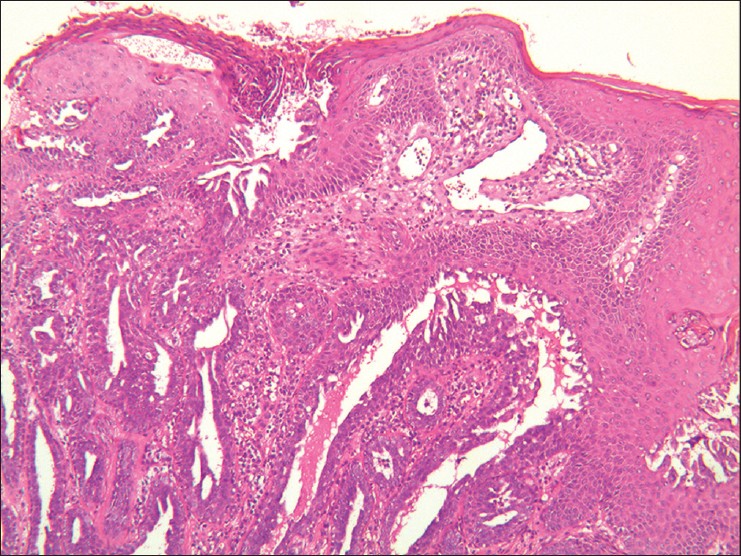 |
| Figure 2: A non-encapsulated tubular mass lined with basal cuboidal myoepithelial cells, and inner columnar epithelium with papillary proliferation and decapitation secretion into the lumen. The stroma was infiltrated with lymphocytes and plasma cells. (H and E, ×40) |
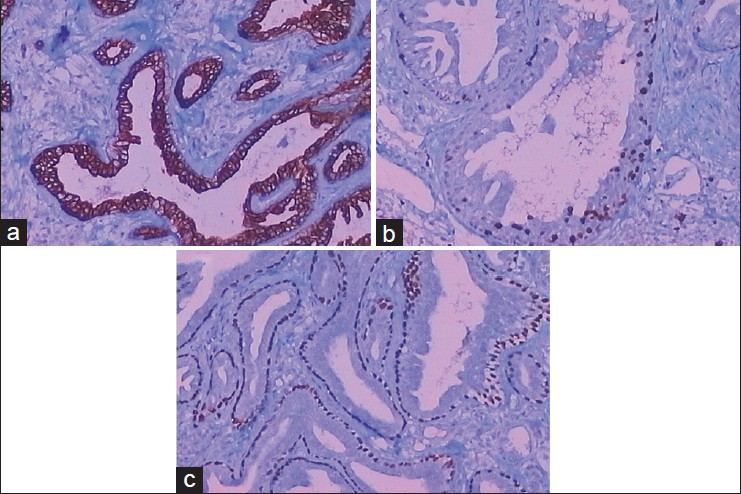 |
| Figure 3: The inner cells of the tubular mass were positive to CK7 (Figure 3a, 100×) and S-100, 10% positive to Ki67 (Figure 3b, 100×), but negative to epithelial membrane antigen (EMA) and estrogen receptor (ER). The outer myoepithelial cells were positive to p63 (Figure 3c, 100×) and negative to p53 |
This uncommon, benign neoplasm of the breast lactiferous ducts usually affects middle-aged women. A few male cases of erosive adenomatosis of the nipple have been reported in English and Chinese literature. [1],[2],[3]
The neoplasm often displays a unilateral eczematous lesion with polymorphic presentation, which is easily confused with Paget′s disease. However, at the later stages, erosive adenomatosis of the nipple is characterized by palpable nodules and induration, whereas Paget′s disease displays more ulceration and destruction of nipple-areola complex. Itching, irritation or burning pains are common symptoms. Histopathological examination reveals a non-enveloped, but bordered tubular and/or papillary mass, which usually extends from the epidermis into the deeper dermis. Rosen and Caicco found four histological growth patterns of the proliferation: Adenosis, papillomatosis, sclerosing papillomatosis, and the mixture. [4] Our case showed a gland-like proliferation with papillary epithelial hyperplasia, which conforms to the mixed pattern. Immunohistochemically, the inner columnar apocrine cells of the duct are often CK7 and S-100 positive, and the outer myoepithelial cells p63, muscle-specific actin (MSA) and calponin positive. Markers of malignancy such as p53, CEA, Ki67, and C-erB-2, aid in the differential diagnosis of breast carcinoma. Our case showed 10% positivity with Ki67, while ER and EMA were negative, indicating a low degree of proliferation of the apocrine epithelium, which conforms to previous reports. [2],[5] Malignant features such as cytologic atypia and invasive growth pattern were not seen in our patient.
Nonetheless, coexistent carcinoma and malignant changes have been reported, [4],[5] so complete excision and long-term follow-up are warranted.
| 1. |
Ishii N, Kusuhara M, Yasumoto S, Hashimoto T. Adenoma of the nipple in a Japanese man. Clin Exp Dermatol 2007;32:448-9.
[Google Scholar]
|
| 2. |
Fernandez-Flores A, Suarez-Penaranda JM. Immunophenotype of nipple adenoma in a male patient. Appl Immunohistochem Mol Morphol 2011;19:190-4.
[Google Scholar]
|
| 3. |
Boutayeb S, Benomar S, Sbitti Y, Harroudi T, Hassam B, Errihani H. Nipple adenoma in a man: An unusual case report. Int J Surg Case Rep 2012;3:190-2.
[Google Scholar]
|
| 4. |
Rosen PP, Caicco JA. Florid papillomatosis of the nipple. A study of 51 patients, including nine with mammary carcinoma. Am J Surg Pathol 1986;10:87-101.
[Google Scholar]
|
| 5. |
Kono S, Kurosumi M, Simooka H, Kawanowa K, Takei H, Suemasu K. Nipple adenoma found in a mastectomy specimen: Report of a case with special regard to the proliferation pattern. Breast Cancer 2007;14:234-8.
[Google Scholar]
|
Fulltext Views
4,247
PDF downloads
3,309





