Translate this page into:
Erythema multiforme-like reaction following COVID-19 vaccination
Corresponding Author: Dr. Biswanath Behera, Department of Dermatology, All India Institute of Medical Sciences, Patrapada, Bhubaneswar, Odisha, India. biswanathbehera61@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kowe PA, Behera B, Sethy M, Viswan P. Erythema multiforme-like reaction following COVID-19 vaccination. Indian J Dermatol Venereol Leprol 2023;89:160.
Sir,
A 40-year-old male presented with eight days history of multiple severely itchy red eruptions all over the body. The lesions started over the extremities 24 hours after the first dose of COVID-19 vaccine (“Covaxin®” a whole–virion inactivated SARS–CoV–2 antigen, Strain: NIV–2020–770). They were not associated with constitutional or systemic features. He consulted a vaccination center and was prescribed cetirizine and calamine lotion without any improvement. He denied a history of recent infections or medications prior to the onset of the skin rash. He did not have a history of previous pneumoccocal or influenza vaccines. There was no history of any systemic involvement. Cutaneous examination revealed multiple erythematous macules, papules, vesicles, and targetoid lesions over the bilateral extremities, trunk and back [Figures 1a to c]. Palm and soles were not affected. Other mucocutaneous, general, and systemic examinations were within normal limits. Dermoscopy under polarised mode (Heine Delta 20T, 10×) from targetoid papules showed central brown peppering and peripheral pinkish-white structureless area (a targetoid pattern) [Figure 2]. His laboratory investigations, complete blood count, erythrocyte sedimentation rate, C-reactive protein, liver function test, and serum urea and creatinine were within normal limits. Histopathology (from a vesicle and a papule) revealed orthokeratosis, spongiosis, intra-epidermal vesicle and focal basal cell hydropic degeneration. The dermis showed moderate-to-severe oedema and perivascular lymphocytes along with a few eosinophils and neutrophils [Figures 3a and b]. Due to the predominant spongiotic reaction pattern and the absence of significant basal vacuolar degeneration and necrotic keratinocytes, the diagnosis of erythema multiforme-like reaction was made. Naranjo scale of adverse drug reaction probability was used to assess the causality; the score was 4, confirming that the vaccine possibly induced the erythema multiforme-like reaction.1 This adverse event was reported to the Pharmacovigilance Programme of India. According to the World Health Organization-Uppsala Monitoring Centre criteria, it was certainly an adverse event secondary to the vaccine (Pharmacovigilance Programme of India Report No. In-IPC-300547804).1 Based on the modified Hartwig and Siegel severity assessment scale, this event comes under the moderate category (level 3).2 The patient was started on prednisolone 30 mg/day, following which he noticed a significant improvement in skin lesions in one week. The patient was counselled to carry a drug card and advised to abstain from the second dose of the same COVID-19 vaccine.
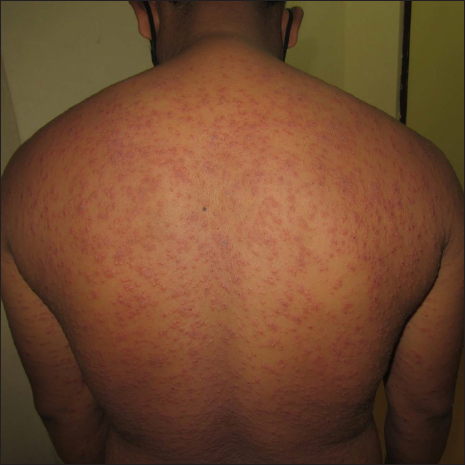
- Multiple erythematous macules, papules, vesicles and targetoid lesions on the back
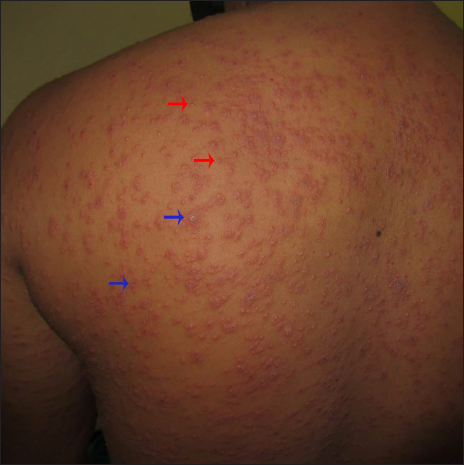
- A closer view shows multiple targetoid lesions (blue arrows) and vesicles (red arrows)
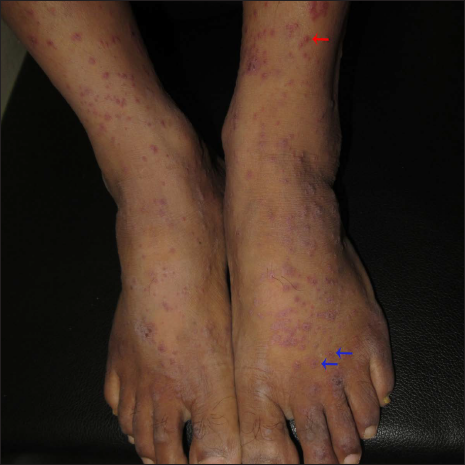
- Multiple erythematous papules, vesicles (red arrow), and targetoid papules (blue arrows)
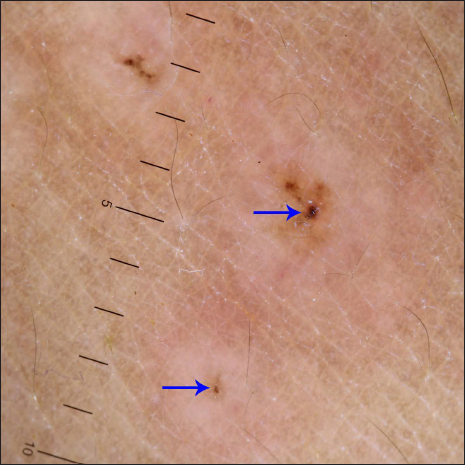
- Dermoscopy under polarised mode (Heine Delta 20T, ×10 magnification) from targetoid papules shows a targetoid pattern comprising central brown peppering (arrows) and peripheral pinkish-white structureless area
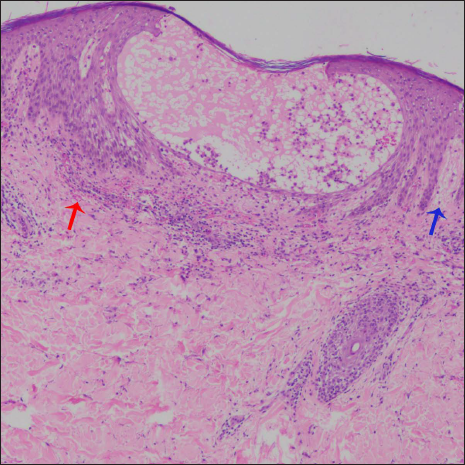
- Histopathology shows spongiosis, intraepidermal vesicle, focal basal cell hydropic degeneration, erythrocyte extravasation (red arrow), and superficial dermal oedema (blue arrow) (H & E, ×100)
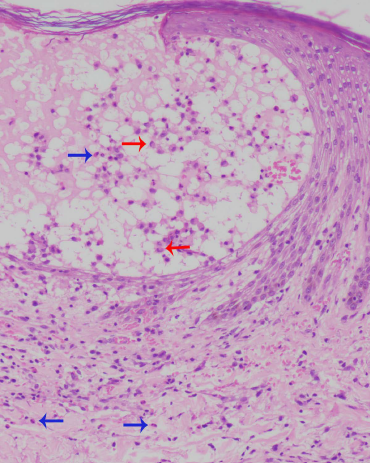
- Erythrocyte extravasation and eosinophils (blue arrows). Red arrows point to the Langerhans cells (H & E, ×400)
Immunisation with the currently available COVID-19 vaccine is the most important and effective measure in controlling this pandemic. However, there is an increase in the emergence of vaccine-related adverse reactions. Both active and inactive content of the vaccine can lead to these events. These include vaccine ingredients such as inactivated or killed viruses and their antigens, conjugating agents, preservatives, stabilisers, adjuvants, antimicrobial agents, excipients (inert substances increasing the bulk of the vaccine) and culture medium used during processing of vaccine.3
Erythema multiforme is a cutaneous hypersensitivity reaction pattern in response to infections and drugs.4 Reports of erythema multiforme following vaccination are infrequent.5 As per Lavery et al. post-infection and post-vaccine induced erythema multiforme have the same etiopathogenesis.4 He hypothesised that erythema multiforme associated with infection is due to a cell-mediated immune response.4 In the index case, the inactivated virion present in the vaccine and its antigen expressed on the surface of keratinocytes possibly resulted in the stimulation of helper-T cells and release of cytokines such as interferon-gamma leading to inflammation. It is known that excipient induced allergic reactions are usually localised to the injection site and are less severe, whereas viral antigen-induced reactions are generalised in distribution and more severe. The second scenario was observed in our case.5
In conclusion, we report a case of erythema multiforme-like reaction following the COVID-19 vaccine. In today’s era of emerging cutaneous adverse drug reactions following COVID-19 vaccination, every case should be reported to the pharmacovigilance department to increase awareness among treating physicians.
Acknowledgement
We acknowledge Mrs. Pinki Mohanty, Pharmacovigilance associate, Indian Pharmacopoeia Commission, NCC-PvPI, Ghaziabad, for helping us register the adverse reaction to COVID-19 vaccine.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- A study of agreement between the Naranjo algorithm and WHO-UMC criteria for causality assessment of adverse drug reactions. Indian J Pharmacol. 2014;46:117-20.
- [CrossRef] [PubMed] [Google Scholar]
- Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229-32.
- [PubMed] [Google Scholar]
- Allergic reactions to current available COVID-19 vaccinations: Pathophysiology, causality, and therapeutic considerations. Vaccines (Basel). 2021;9:221.
- [CrossRef] [PubMed] [Google Scholar]
- A flare of pre-existing erythema multiforme following BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine. Clin Exp Dermatol. 2021;46:1325-7.
- [CrossRef] [PubMed] [Google Scholar]
- Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85:2694-706.
- [CrossRef] [PubMed] [Google Scholar]





