Translate this page into:
Evidence-based treatments for pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid: A systematic review
Correspondence Address:
Sanjay Singh
C-23, Swastik Towers, Lanka, Varanasi, Uttar Pradesh
India
| How to cite this article: Singh S. Evidence-based treatments for pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid: A systematic review. Indian J Dermatol Venereol Leprol 2011;77:456-469 |
Abstract
Background: Pemphigus, bullous pemphigoid, and epidermolysis bullosa acquisita are autoimmune diseases of skin associated with considerable morbidity and sometimes mortality. There is no cure for these diseases. Aims: To summarize evidence-based treatments for these diseases by performing a systematic review. Methods: The research protocol included the following steps: identification of databases to be searched, defining search strategy, searching the databases for references, first-stage screening of the abstracts, second-stage screening of full texts of articles identified after the first-stage screening, data extraction from the identified articles after second-stage screening, quality appraisal of the studies using the Delphi list, and summarizing the findings. Results: No randomized controlled trials of interventions in pemphigus vegetans, pemphigus erythematosus, and epidermolysis bullosa acquisita could be found. After the second-stage screening, 12 randomized controlled trials were analyzed, which included patients with pemphigus vulgaris or pemphigus vulgaris and pemphigus foliaceus, and 7 which included patients with bullous pemphigoid. Conclusions: Number of high-quality randomized controlled trials conducted on pemphigus and bullous pemphigoid is small. Oral corticosteroid along with a steroid-sparing agent appears to be the most effective treatment for pemphigus. Azathioprine may be most effective as a steroid-sparing agent. Topical corticosteroid therapy (as studied) is effective for bullous pemphigoid and appears to be superior to oral corticosteroid for extensive disease. Some suggestions about future research are made.Introduction
Pemphigus is a group of autoimmune diseases of skin and mucous membranes, which is characterized by autoantibodies directed against antigens desmogleins 1 and/or 3 in the epidermis. This results in acantholysis in epidermis and clinically in the formation of flaccid blisters. There are mainly two types of pemphigus, pemphigus vulgaris (and its variant pemphigus vegetans), and pemphigus foliaceus (and its variant pemphigus erythematosus). The diseases are associated with considerable morbidity and sometimes mortality. Use of systemic glucocorticoids and other immunosuppressive drugs has changed the outlook in a large proportion of patients, but presently there is no cure of pemphigus.
Bullous pemphigoid is an autoimmune disease of skin usually occurring in the elderly. It is characterized by autoantibodies against the 180-kd (BP 180) and/or 230-kd (BP 230) molecules present in basal keratinocyte hemidesmosomes in the dermoepidermal junction. This results in split at the dermoepidermal junction and clinically in the formation of tense blisters. Pemphigoid is associated with considerable morbidity and sometimes mortality. Corticosteroids, topical or systemic, and sometimes other immunosuppressive agents help many patients, but presently there is no cure.
Epidermolysis bullosa acquisita (EBA) is a rare autoimmune disease characterized by skin fragility and subepidermal blisters due to the formation of autoantibodies against type VII collagen within the anchoring fibrils at the dermoepidermal junctions. EBA is associated with considerable morbidity.
In the present review, an attempt will be made to answer the question: what are the evidence-based (randomized controlled trials-based) treatments for pemphigus, bullous pemphigoid, and epidermolysis bullosa acquisita?
Methods
Pemphigus
The research protocol included the following steps: identification of databases to be searched, defining search strategy, searching the databases for references, first-stage screening of the abstracts, second-stage screening of full texts of articles identified after the first-stage screening, data extraction from the identified articles after second-stage screening, quality appraisal of the studies, and summarizing the findings.
Databases searched
Following two databases were searched:
- PubMed [ http://www.PubMed.gov ( http://www.ncbi.nlm.nih.gov/pubmed/ )].
- Cochrane Central Register of Controlled Trials (Clinical Trials) ( http://onlinelibrary.wiley.com/o/cochrane/cochrane_clcentral_articles_fs.html ).
Search strategy
1. PubMed: This was searched for the phrases "pemphigus vulgaris," "pemphigus foliaceus," "pemphigus vegetans," and "pemphigus erythematosus" separately by activating the limit "Clinical Trial" and using the search field tag "Title/Abstract."
2. Cochrane Central Register of Controlled Trials: Search was performed for the above diseases separately in "Title, Abstract, or Keywords."
The search was first performed on November 9, 2010 and was repeated on November 28, 2010; both searches resulted in identical references. All the articles thus identified went into first-stage screening.
First-stage screening
Abstracts of all the articles identified in the above-mentioned databases were read. Only those abstracts were selected for the second-stage screening, which met all of the following three inclusion criteria: (a) human trial, (b) prospective trial, and (c) controlled trial.
Second-stage screening
This was performed on the full-text articles. Full-texts of the articles which met the first-stage screening criteria were obtained. Only those articles were selected which met both of the following selection criteria: (a) mention of randomization in methods and (b) mention in methods that at least one of the following three tests were performed: (i) direct immunofluorescence test for detection of immunoglobulin G (IgG) on keratinocyte cell surface, (ii) test for detection of antibodies against desmoglein 1 and/or 3, or (iii) indirect immunofluorescence test for detecting IgG in patient′s serum, which binds the cell surface of normal keratinocytes.
Articles that met the above-mentioned criteria were the randomized controlled trials (RCTs) of interventions in patients with pemphigus and these went into the data extraction stage.
Data extraction
Full-texts of the articles were read and the data regarding the following variables was noted separately for each article: name of disease(s) with which the patients were affected, number of centers where the trial was conducted and name of the country, interventions, adverse events, efficacy, and conclusions.
Quality appraisal
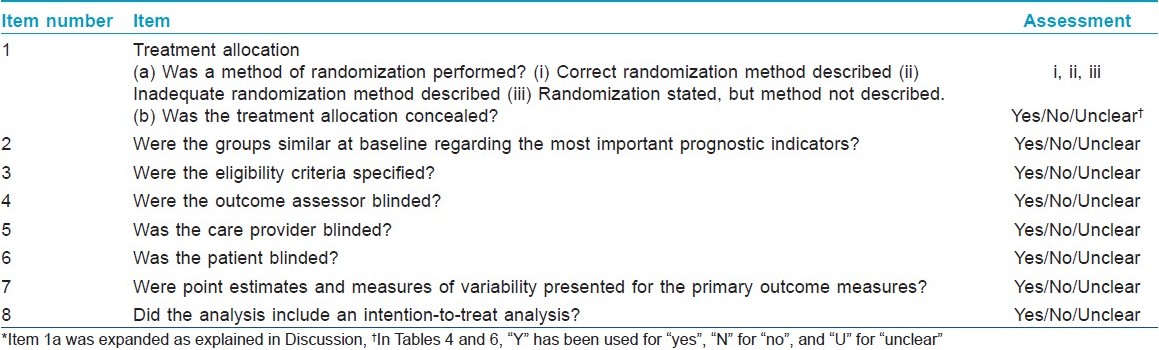
Quality appraisal of the RCTs was done by using the Delphi List, [1] which was expanded with respect to item number 1a as explained in the Discussion [Table - 1].
Summarizing the findings
Summary of the RCTs was presented in tabular format.
Bullous pemphigoid
The same research protocol was followed as described above for pemphigus, with the following changes: Databases were searched for the word "pemphigoid." In the second-stage screening, only the articles that met both of the following selection criteria were selected: (a) mention of randomization in methods and (b) diagnosis of bullous pemphigoid by at least one of the following tests: (i) positive direct immunofluorescence test for C3 and/or IgG at the dermoepidermal junction, (ii) serum IgG labeling epidermal roof by indirect immunofluorescence, (iii) detection of antibodies against BP180 and/or BP230 antigens, or (iv) demonstration by immunoelectron microscopy of deposition of IgG associated with basal cell hemidesmosomes.
Epidermolysis bullosa acquisita
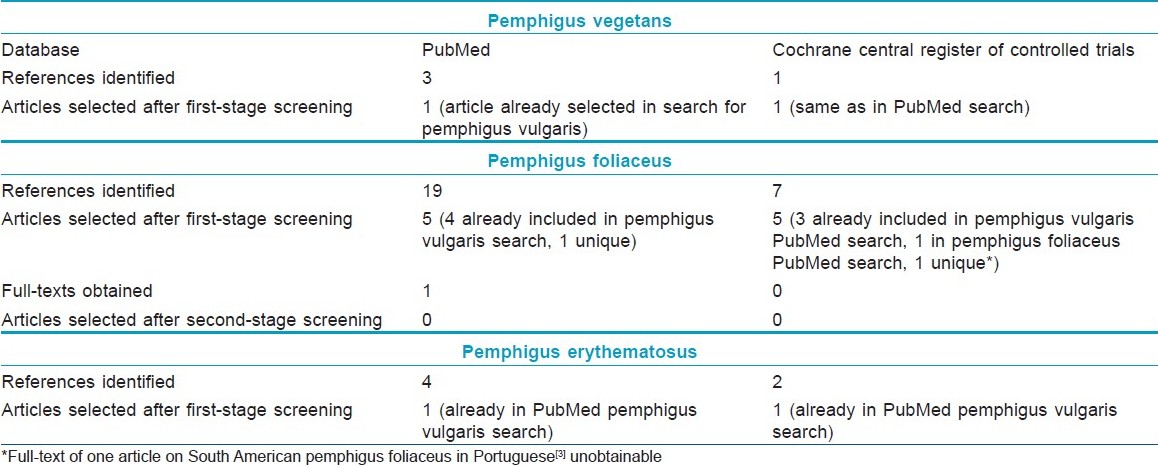
The same research protocol was followed as described above for pemphigus. Databases were searched for the phrase "epidermolysis bullosa acquisita." PubMed search resulted in three references which were excluded in first-stage screening. Search of Cochrane Central Register of Controlled Trials did not result in any reference.
As no RCTs were available on epidermolysis bullosa acquisita, no RCT-based conclusions can be drawn about its treatment.
Results
Pemphigus
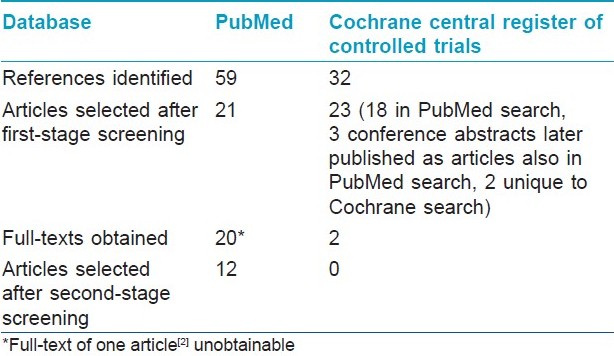
Number of articles on pemphigus vulgaris selected at different stages of the review is shown in [Table - 2] and those related to other types of pemphigus in [Table - 3]. On reading the full-texts of articles, it became clear that no RCTs of interventions exclusively in pemphigus vegetans, pemphigus foliaceus, or pemphigus erythematosus were available. Of the 12 selected RCTs, 8 included patients with pemphigus vulgaris only and 4 included patients with both pemphigus vulgaris and pemphigus foliaceus. None of the available RCTs were found to include patients with pemphigus vegetans or pemphigus erythematosus.
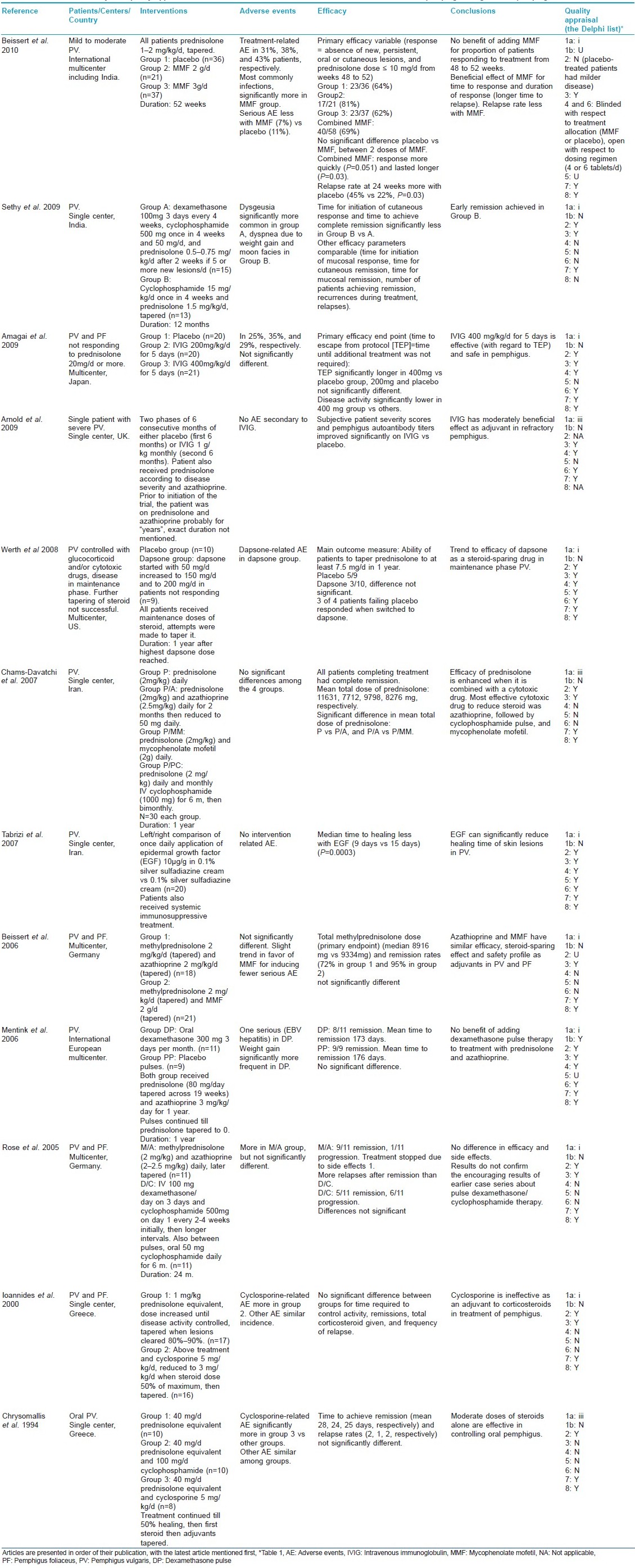
Summary of the selected articles [4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15] of interventions in pemphigus vulgaris and foliaceus is presented in [Table - 4].
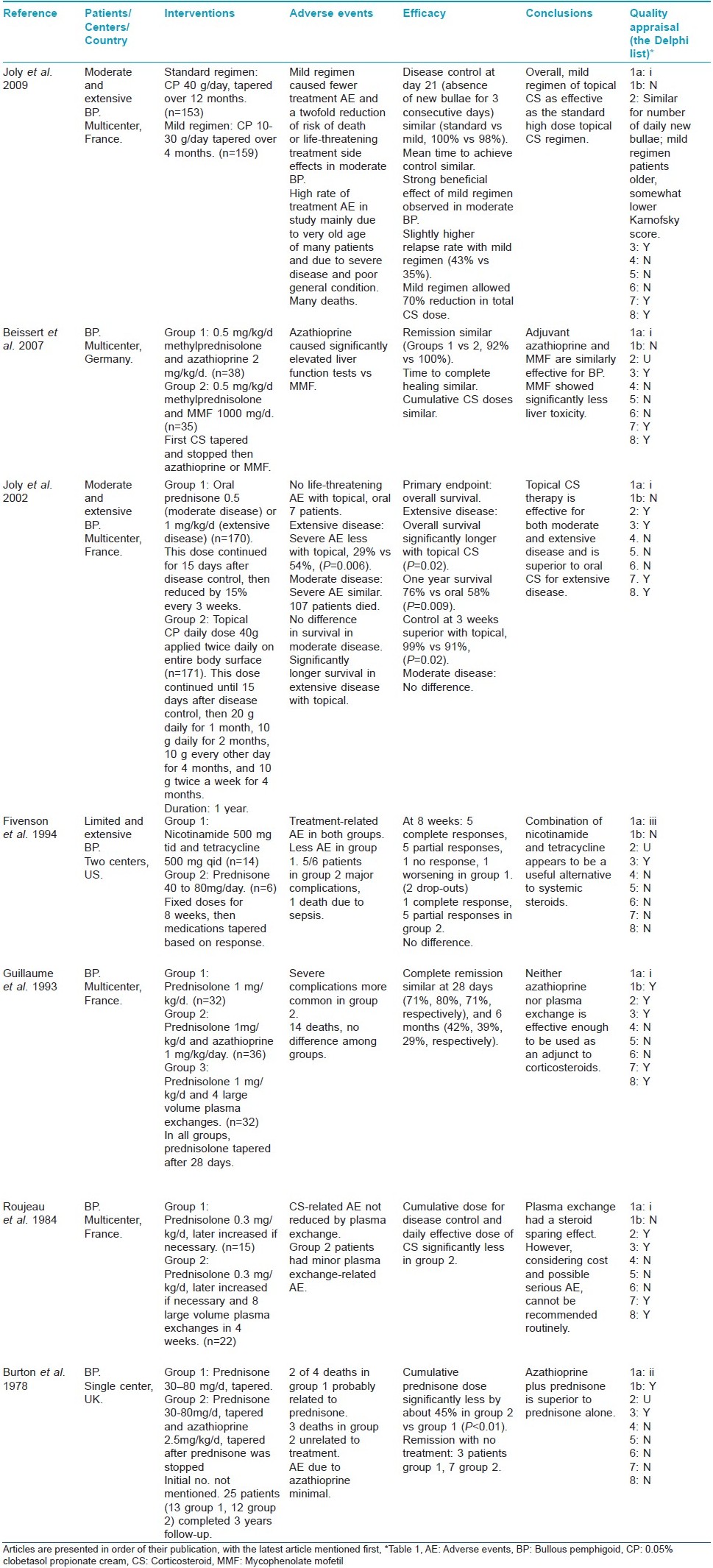
Bullous pemphigoid
Number of articles on bullous pemphigoid selected at different stages of the systematic review is shown in [Table - 5]. Seven articles [19],[20],[21],[22],[23],[24],[25] met the selection criteria of second-stage screening and were selected for final analysis. Results are presented in [Table - 6].

Discussion
Pemphigus
In the present review, an attempt was made to find out the evidence-based treatment for pemphigus. Good quality evidence consists of results of randomized controlled trials (RCTs). When an initial search was made on the two databases by using the phrase "randomized controlled trial" and name of a disease (eg, pemphigus vulgaris), it was found that very few articles were identified. The reason for this finding was that usually the articles that reported RCTs did not have this phrase in the titles or abstracts. Therefore, the search strategy was modified and it included a two-stage screening. It appears that this modified plan led to the identification of most, if not all, of the relevant articles.
PubMed is a service of the United States National Library of Medicine and the National Institutes of Health and comprises more than 20 million citations for biomedical literature from MEDLINE, life science journals and online books. Approximately 5400 journals published in more than 80 countries are currently indexed in MEDLINE. The other database selected was the Cochrane Central Register of Controlled Trials. This database includes details of articles from MEDLINE and also from EMBASE and other published and unpublished sources. EMBASE currently has over 23 million indexed records from more than 7500 journals.
For the second-stage screening, full-texts of 23 of 25 articles on pemphigus vulgaris and pemphigus foliaceus selected after the first-stage screening were obtained, from which 12 articles were finally selected [Table - 2] and [Table - 3]. These 12 studies used randomization for allocating treatments to different groups of patients. Most of the articles used at least one of the three immunological tests mentioned in methods of this article for diagnosing pemphigus. Two articles, [5],[15] which appeared to be relevant, mentioned that immunological tests (enzyme-linked immunosorbent assay [ELISA] for antidesmoglein 1 and 3 antibodies [5] and direct and indirect immunofluorescence assays [15] ) were performed, but it was unclear to this reviewer how the results of these tests were used in making the diagnosis.
It may be very important to have clear-cut diagnostic criteria for pemphigus. This is relevant in individual patients as well as in a situation when a patient may be included in a clinical study. Using a uniform set of criteria will make it easier for results of different clinical studies to be compared. One of the articles selected in this review used a set of diagnostic criteria, which appear to be appealing. [6] These Japanese diagnostic criteria are as follows: pemphigus is diagnosed when at least one item from every three findings, or two items from clinical findings and one item from immunological findings are satisfied. The three groups of findings are:
- Clinical findings (multiple, easily rupturing, flaccid blisters of the skin; subsequent progressive, refractory erosions or crusts after blisters; noninfectious blisters or erosions of visible mucosa including oral mucosa; Nikolsky sign)
- Histologic findings (intraepidermal blisters caused by acantholysis).
- Immunologic findings (IgG or complement deposition in the intercellular spaces of the lesional or normal-appearing skin and mucosa detected by direct immunofluorescence antibody assay; antidesmoglein antibody identified by indirect fluorescent antibody assay or ELISA).
In the immunologic findings, indirect immunofluorescence test for detecting IgG in patient′s serum which binds the cell surface of normal keratinocytes may also be added. Scientifically, one would require a set of diagnostic criteria for which sensitivity and specificity have been worked out.
Assessment of the quality of the RCTs is a key step in a systematic review. Several quality scales have been developed for this purpose. In the present review, quality assessment was done using the Delphi list [Table - 1], which is a criteria list for quality assessment of RCTs specially for conducting systematic reviews. [1] This list consists of eight items and item one was further elaborated for quality assessment in this review. The first item of the original Delphi list is as follows: Treatment allocation (a) was a method of randomization performed? (b) was the treatment allocation concealed? Item 1a was expanded to give three possible responses: (i) Correct randomization method described, (ii) Inadequate randomization method described, and (iii) Randomization stated, but method not described. This expansion provided a clearer picture about the randomization procedure. Treatment allocation concealment, which is considered to be the most important indicator of quality of a trial, was understood to have taken place only when there was a clear statement about it or when there was a statement which meant that treatment to be allocated was not known before the patient was entered into the study. Quality appraisal of RCTs is sometimes done to produce a quality score and a threshold score may be used for inclusion of RCTs in a systematic review. However, as there may be differences of opinion among the reviewers with regard to the relative importance of different items of quality, in the present review detailed data about different quality items of all selected articles was presented [Table - 4].
The Cochrane Collaboration publishes high-quality systematic reviews. Its review on interventions for pemphigus vulgaris and pemphigus foliaceus [26] describes 11 RCTs, using a different search strategy. Eight of these RCTs were identified in the present review also; four articles identified in the present review [4],[5],[6],[7] are not included in the Cochrane review. On the other hand, three articles [27],[28],[29] included in the Cochrane review were not identified in the database search for this review. These articles described the use of glucocorticoid alone versus glucocorticoid plus a traditional Chinese medicine, [27] low (0.5 mg/kg/day) versus high (1.0 mg/kg/day) initial doses of prednisolone, [28] and oral prednisolone versus oral prednisolone and plasma exchange. [29] All these studies had serious methodological problems and the effects of study interventions were considered inconclusive. [26]
Following general conclusions may be drawn about the evidence-based treatment of pemphigus from the present review:
- Number of RCTs conducted on pemphigus is small. Common important shortcomings of these RCTs are: absence of blinding, no mention of treatment allocation concealment, and small sample size.
- The diseases included in these RCTs are pemphigus vulgaris and pemphigus foliaceus.
- Oral glucocorticoid along with a steroid-sparing agent appears to be the most effective treatment (two RCTs). [4],[9]
- Most effective steroid-sparing drug appears to be azathioprine (one RCT). [9]
- Mycophenolate mofetil (MMF) may have similar (one RCT) [11] or less (one RCT) [9] steroid-sparing effect and similar safety profile compared to azathioprine (two RCTs) [9],[11] or mild steroid-sparing effect (one RCT). [4]
- There appears to be no benefit of adding dexamethasone pulse therapy to treatment with prednisolone and azathioprine (one RCT). [12]
- Dexamethasone and cyclophosphamide pulse therapy as tested may be similar in efficacy to methylprednisolone and azathioprine regimen (one RCT). [13]
- Intravenous immunoglobulin (IVIg) may have moderate effect as an adjuvant (one RCT) [7] or alone (one RCT) [6] on treatment-resistant pemphigus.
- There may be a trend to some efficacy of dapsone as a steroid-sparing drug in maintenance phase pemphigus vulgaris (one RCT). [8]
- Moderate doses of glucocorticoids without other immunosuppressive agent may be effective in controlling oral pemphigus (one RCT). [15]
- Epidermal growth factor may reduce healing time of skin lesions in pemphigus vulgaris (one RCT). [10]
- Cyclosporine may be ineffective as a steroid-sparing agent (one RCT). [14]
In view of the foregoing discussion, following suggestions may be made about future research on treatment of pemphigus:
- Selection of patients for RCTs may preferably be based on uniform diagnostic criteria.
- Selection criteria may preferably include severity assessment of the disease. Also, validated severity scale will help in assessing response to treatment. Two proposed scales, autoimmune bullous skin disorder intensity score (ABSIS) [30] and pemphigus disease area index (PDAI), [31] have recently been compared [32] for inter- and intra-rater reliability.
- In an RCT, patients with one type of pemphigus may only be preferably included.
- RCTs are required to compare the efficacy and safety of different doses of glucocorticoids used with different steroid-sparing agents.
- Long-term follow-up of patients included in RCTs is important to find out relapse rate after remission with different treatments.
- The issue of maintenance therapy to prevent relapse after remission may also preferably be addressed.
- Effect of different treatments on the quality of life of patients with pemphigus may also be studied.
Bullous pemphigoid
In the second-stage screening, initially the first test in the second criteria was kept as follows: (i) positive direct immunofluorescence test for C3 and/or IgG on the epidermal roof of salt-split skin. This was done so that the patients with bullous pemphigoid are differentiated from those with EBA. But it was found that none of the articles in the second-stage screening met any of the criteria (ii), (iii), or (iv) and in only one article [20] the diagnosis was made by detection of autoantibody deposition at the blister roof on salt-split skin. Therefore, as a compromise, the wordings of the first test were changed to "positive direct immunofluorescence test for C3 and/or IgG at the dermoepidermal junction." It is to be clarified that in six [19],[21],[22],[23],[24],[25] of the seven RCTs, which were selected for final analysis based on this criteria, the possibility of inadvertent inclusion of some patients with EBA cannot be ruled out.
This brings us to a situation similar to pemphigus. There are no uniform diagnostic criteria available for making diagnosis of bullous pemphigoid, which are used for individual patients and for their inclusion in clinical studies. It is important to have clear-cut diagnostic criteria for bullous pemphigoid, which include at least one positive immunological test from the following four tests: (i) positive direct immunofluorescence test for C3 and/or IgG on the epidermal roof of salt-split skin, (ii) serum IgG labeling epidermal roof by indirect immunofluorescence, (iii) detection of antibodies against BP180 and/or BP230 antigens, or (iv) demonstration by immunoelectron microscopy of deposition of IgG associated with basal cell hemidesmosomes.
Cochrane systematic review on interventions for bullous pemphigoid describes 10 RCTs. [33] The three extra articles in the Cochrane review were identified in search of databases for the present review also. One article was in Chinese [16] and the other two in French [17],[18] and their full texts were unobtainable. These studies compared prednisolone alone versus prednisolone plus a Chinese medicine, [16] methylprednisolone versus prednisolone, [17] and higher versus lower doses of prednisolone. [18] All the three studies had important methodological problems and the results did not show statistically significant differences in any study. [33]
Following general conclusions may be drawn about the evidence-based treatment of bullous pemphigoid from the present review:
- Number of RCTs conducted on bullous pemphigoid is small. None of the studies identified in this review were blinded and in only a few studies treatment allocation was concealed.
- Topical corticosteroid therapy is effective for both moderate and extensive disease and appears to be superior to oral corticosteroid for extensive disease (one RCT). [21] Low doses of topical corticosteroid may also be effective (one RCT). [19]
- Adding azathioprine to oral corticosteroid may (one RCT) [25] or may not (one RCT) [23] be superior to oral corticosteroid alone.
- Adding plasma exchange to oral corticosteroid may (one RCT) [24] or may not (one RCT) [23] be superior to oral corticosteroid alone.
- Adjuvant azathioprine and MMF may be similarly effective. MMF may have significantly less liver toxicity (one RCT). [20]
- Combination of nicotinamide and tetracycline appears to be a useful alternative to systemic steroids (one RCT). [22]
In the light of the foregoing discussion, following suggestions may be made regarding future research on treatment of bullous pemphigoid:
- Selection of patients for RCTs may preferably be based on uniform diagnostic criteria, which also enable exclusion of patients with EBA.
- Selection criteria may preferably include severity assessment of the disease. Acceptable severity assessment scale may preferably be developed.
- More RCTs are required to confirm the promising efficacy of different doses of topical corticosteroid therapy versus oral corticosteroid therapy.
- Different doses of oral corticosteroids may be evaluated in RCTs to find out the safest effective dose.
- RCTs are required to find out effective steroid-sparing agents with favorable toxicity profile.
- Efficacy of combination of nicotinamide and tetracycline may be studied as a useful alternative to systemic corticosteroids.
- Long-term follow-up of patients included in RCTs is important to find out relapse rate after remission with different treatments.
- The issue of maintenance therapy to prevent relapse after remission may also preferably be addressed.
- Effect of different treatments on the quality of life of patients with bullous pemphigoid may also be studied.
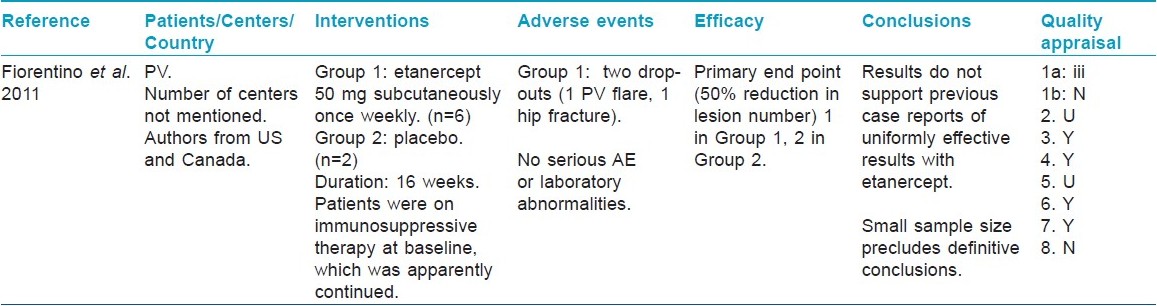
At the final proof reading stage, repeat search on June 10, 2011 found 3 new articles on pemphigus vulgaris in PubMed and Cochrane Central Register of Controlled Trials each. Two articles were same in both databases. Only one article [34] passed through the second-stage screening [Table - 7]. One new article on bullous pemphigoid found in PubMed was excluded in first-stage screening. No new articles were found on other diseases.
| 1. |
Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235-41.
[Google Scholar]
|
| 2. |
Akhtar SJ, Hasan MU. Treatment of pemphigus: A local experience. J Pak Med Assoc 1998;48:300-4.
[Google Scholar]
|
| 3. |
Auad A, Auad T, Auad A, Auad P. Co-adjuvant therapy with auranofin in the treatment of the South American pemphigus foliaceus (a double blind study). An Bras Dermatol 1986;61:131-4.
[Google Scholar]
|
| 4. |
Beissert S, Mimouni D, Kanwar AJ, Solomons N, Kalia V, Anhalt GJ. Treating pemphigus vulgaris with prednisone and mycophenolate mofetil: A multicenter, randomized, placebo-controlled trial. J Invest Dermatol 2010;130:2041-8.
[Google Scholar]
|
| 5. |
Sethy PK, Khandpur S, Sharma VK. Randomized open comparative trial of dexamethasone-cyclophosphamide pulse and daily oral cyclophosphamide versus cyclophosphamide pulse and daily oral prednisolone in pemphigus vulgaris. Indian J Dermatol Venereol Leprol 2009;75:476-82.
[Google Scholar]
|
| 6. |
Amagai M, Ikeda S, Shimizu H, Iizuka H, Hanada K, Aiba S, et al. A randomized double-blind trial of intravenous immunoglobulin for pemphigus. J Am Acad Dermatol 2009;60:595-603.
[Google Scholar]
|
| 7. |
Arnold DF, Shine BB, Wojnarowska F, Misbah SA. An "n-of-1" placebo-controlled crossover trial of intravenous immunoglobulin as adjuvant therapy in refractory pemphigus vulgaris. Br J Dermatol 2009;160:1098-102.
[Google Scholar]
|
| 8. |
Werth VP, Fiveson D, Pandya AG, Chen D, Rico MJ, Albrecht J, et al. Multicenter randomized, double-blind, placebo-controlled, clinical trial of dapsone as a glucocorticoid-sparing agent in maintenance-phase pemphigus vulgaris. Arch Dermatol 2008;144:25-32.
[Google Scholar]
|
| 9. |
Chams-Davatchi C, Esmaili N, Daneshpazhooh M, Valikhani M, Balighi K, Hallaji Z, et al. Randomized controlled open-label trial of four treatment regimens for pemphigus vulgaris. J Am Acad Dermatol 2007;57:622-8.
[Google Scholar]
|
| 10. |
Tabrizi MN, Chams-Davatchi C, Esmaeeli N, Noormohammadpoor P, Safar F, Etemadzadeh H, et al. Accelerating effects of epidermal growth factor on skin lesions of pemphigus vulgaris: A double-blind, randomized, controlled trial. J Eur Acad Dermatol Venereol 2007;21:79-84.
[Google Scholar]
|
| 11. |
Beissert S, Werfel T, Frieling U, Bohm M, Sticherling M, Stadler R, et al. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of pemphigus. Arch Dermatol 2006;142:1447-54.
[Google Scholar]
|
| 12. |
Mentink LF, Mackenzie MW, Toth GG, Laseur M, Lambert FP, Veeger NJ, et al. Randomized controlled trial of adjuvant oral dexamethasone pulse therapy in pemphigus vulgaris. PEMPULS trial. Arch Dermatol 2006;142:570-6.
[Google Scholar]
|
| 13. |
Rose E, Wever S, Zillikens D, Linse R, Haustein UF, Brocker EB. Intravenous dexamethasone-cyclophosphamide pulse therapy in comparison with oral methylprednisolone-azathioprine therapy in patients with pemphigus vulgaris: Results of a multicenter prospectively randomized study. J Dtsch Dermatol Ges 2005;3:200-6.
[Google Scholar]
|
| 14. |
Ioannides D, Chrysomallis F, Bystryn JC. Ineffectiveness of cyclosporine as an adjuvant to corticosteroids in the treatment of pemphigus. Arch Dermatol 2000;136:868-72.
[Google Scholar]
|
| 15. |
Chrysomallis F, Ioannides D, Teknetzis A, Panagiotidou D, Minas A. Treatment of oral pemphigus vulgaris. Int J Dermatol 1994;33:803-7.
[Google Scholar]
|
| 16. |
Liu BG, Li ZY, Du M. Effects of jingui shenqi pill combined prednisone on expression of glucocorticoid receptor and its clinical effect in treating bullous pemphigoid patients. Zhongguo Zhong Xi Yi Jie He Za Zhi 2006;26:881-4.
[Google Scholar]
|
| 17. |
Dreno B, Sassolas B, Lacour P, Montpoint S, Lota I, Giordano F, et al. Methylprednisolone versus prednisolone methylsulfobenzoate in pemphigoid: A comparative multicenter study. Ann Dermatol Venereol 1993;120:518-21.
[Google Scholar]
|
| 18. |
Morel P, Guillaume JC. Treatment of bullous pemphigoid with prednisolone only: 0.75 mg/kg/day versus 1.25 mg/kg/day. A multicenter randomized study. Ann Dermatol Venereol 1984;111:925-8.
[Google Scholar]
|
| 19. |
Joly P, Roujeau JC, Benichou J, Delaporte E, D'Incan M, Dreno B, et al. A comparison of two regimens of topical corticosteroids in the treatment of patients with bullous pemphigoid: A multicenter randomized study. J Invest Dermatol 2009;129:1681-7.
et al. A comparison of two regimens of topical corticosteroids in the treatment of patients with bullous pemphigoid: A multicenter randomized study. J Invest Dermatol 2009;129:1681-7.'>[Google Scholar]
|
| 20. |
Beissert S, Werfel T, Frieling U, Bohm M, Sticherling M, Stadler R, et al. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of bullous pemphigoid. Arch Dermatol 2007;143:1536-42.
[Google Scholar]
|
| 21. |
Joly P, Roujeau JC, Benichou J, Picard C, Dreno B, Delaporte E, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med 2002;346:321-7.
[Google Scholar]
|
| 22. |
Fivenson DP, Breneman DL, Rosen GB, Hersh CS, Cardone S, Mutasim D. Nicotinamide and tetracycline therapy of bullous pemphigoid. Arch Dermatol 1994;130:753-8.
[Google Scholar]
|
| 23. |
Guillaume JC, Vaillant L, Bernard P, Picard C, Prost C, Labeille B, et al. Controlled trial of azathioprine and plasma exchange in addition to prednisolone in the treatment of bullous pemphigoid. Arch Dermatol 1993;129:49-53.
[Google Scholar]
|
| 24. |
Roujeau JC, Morel P, Dalle E, Guillot B, Gorin I, Lorette G, et al. Plasma exchange in bullous pemphigoid. Lancet 1984;324:486-9.
[Google Scholar]
|
| 25. |
Burton JL, Harman RR, Peachey RD, Warin RP. Azathioprine plus prednisone in treatment of pemphigoid. Br Med J 1978;2:1190-1.
[Google Scholar]
|
| 26. |
Martin LK, Agero AL, Werth V, Villanueva E, Segall J, Murrell DF. Interventions for pemphigus vulgaris and pemphigus foliaceus. Cochrane Database Syst Rev 2009;1:CD006263.
[Google Scholar]
|
| 27. |
Luo X, Zhu L, Tao L, Su W, Fu H, Zheng Z, et al. The clinical and laboratory research for Chinese traditional medicine in the treatment of pemphigus (Chinese). J Clin Dermatol 2003;32:38-41.
[Google Scholar]
|
| 28. |
Ratnam KV, Phay KL, Tan CK. Pemphigus therapy with oral prednisolone regimens. A 5-year study. Int J Dermatol 1990;29:363-7.
[Google Scholar]
|
| 29. |
Guillaume JC, Roujeau JC, Morel P, Doutre MS, Guillot B, Lambert D, et al. Controlled study of plasma exchange in pemphigus. Arch Dermatol 1988;124:1659-63.
[Google Scholar]
|
| 30. |
Pfütze M, Niedermeier A, Hertl M, Eming R. Introducing a novel Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) in pemphigus. Eur J Dermatol 2007; 17: 4-11.
[Google Scholar]
|
| 31. |
Murrell DF, Dick S, Ahmed AR, Amagia M, Barnadas MA, Borradori L, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol 2008; 58: 1043-1046.
[Google Scholar]
|
| 32. |
Rosenbach M, Murrell DF, Bystryn J-C, Dulay S, Dick S, Fakharzadeh S, et al. Reliability and convergent validity of two outcome instruments for pemphigus. J Invest Dermatol 2009; 129: 2404-2410.
[Google Scholar]
|
| 33. |
Kirtschig G, Middleton P, Bennett C, Murrell DF, Wojnarowska F, Khumalo NP. Interventions for bullous pemphigoid. Cochrane Database Syst Rev 2010;10:CD002292.
[Google Scholar]
|
| 34. |
Fiorentino DF, Garcia MS, Rehmus W, Kimball AB. A pilot study of etanercept treatment for pemphigus vulgaris. Arch Dermatol 2011; 147: 117-8.
[Google Scholar]
|
Fulltext Views
7,284
PDF downloads
2,820





