Translate this page into:
Exacerbation of systemic lupus erythematosus in a patient with concomitant chronic plaque psoriasis treated with ustekinumab
Correspondence Address:
Adawiyah Jamil
Department of Medicine, Dermatology Unit, Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur
Malaysia
| How to cite this article: Goh SW, Jamil A, Nor NM, Cader RA, Shaharir SS. Exacerbation of systemic lupus erythematosus in a patient with concomitant chronic plaque psoriasis treated with ustekinumab. Indian J Dermatol Venereol Leprol 2020;86:68-70 |
Sir,
Immunosuppressants and immunomodulators are the mainstay of treatment in systemic lupus erythematosus (SLE) and psoriasis. Ustekinumab, a monoclonal antibody against IL-12 and IL-23, has proven roles in psoriasis. In SLE, its efficacy has been demonstrated in a few cases.[1],[2],[3],[4] We describe a patient with both psoriasis and SLE, who developed exacerbations of cutaneous LE and lupus nephritis following ustekinumab therapy. She presented to the University Kebangsaan Malaysia Medical Centre with acute SLE, at the age of 13 years, with fever, malar rash, photosensitivity, alopecia, arthralgia, mouth ulcers, cytoid bodies, and generalized lymphadenopathy. Laboratory investigations revealed leucopenia, autoimmune hemolytic anemia, thrombocytopenia, ANA 1:640, positive anti-DsDNA, reduced C3 and C4, hematuria 2+, and proteinuria 3+ with urine protein creatinine index of 0.54. She developed immune thrombocytopenic purpura (ITP). While her most systemic problems responded well to cyclosporine and azathioprine, ITP persisted despite these treatments.
At the age of 37 years she developed psoriasiform plaques [Figure - 1] with body surface area (BSA) affected being 55%, Psoriasis Area and Severity Index (PASI) 29.9, and Dermatology Life Quality Index of 22, while on prednisolone 7.5 mg od, cyclosporine 25 mg od, and azathioprine 100mg od for ITP. The doses of prednisolone and cyclosporine were increased and hydroxychloroquine was added, as subacute cutaneous LE was a differential diagnosis. Skin biopsy [Figure - 2] later confirmed psoriasis; direct immunofluorescence was negative for IgA, IgG, and C3. SLE disease activity index (SLEDAI) was low, that is, 4, and erythrocyte sedimentation rate, C3, and C4 were normal with no other systemic involvement. There was no psoriatic arthropathy. She became erythrodermic, while on prednisolone 30 mg od, cyclosporine 75 mg od, and azathioprine 100 mg od. Her psoriasis responded poorly to cyclosporine and she did not tolerate acitretin. Control was eventually achieved (BSA 5%) with methotrexate 15 mg weekly. She developed an episode of acute lupus with musculoskeletal symptoms and cutaneous vasculitis, while on prednisolone 7.5 mg od, hydroxychloroquine 200 mg od, and methotrexate 15 mg weekly. Vasculitis resolved with higher dose of prednisolone. However, it precipitated a flare of psoriasis, upon tapering down of the dosage. Ustekinumab was started once cumulative methotrexate dose reached 3.5 g. After two doses of ustekinumab, PASI reduced from 50 to 0. However, she developed extensive biopsy-proven cutaneous leucocytoclastic vasculitis that required high-dose systemic steroids. After five doses of ustekinumab, she developed acute class IV lupus nephritis with urine protein creatinine index of 0.34. While she was on ustekinumab, she was observed to have more incidences of infections such as urinary tract infections, tinea corporis, and herpes zoster. Her psoriasis was quiescent with aggressive treatment of lupus nephritis that included high-dose systemic steroids and cyclophosphamide, and thus ustekinumab was discontinued.
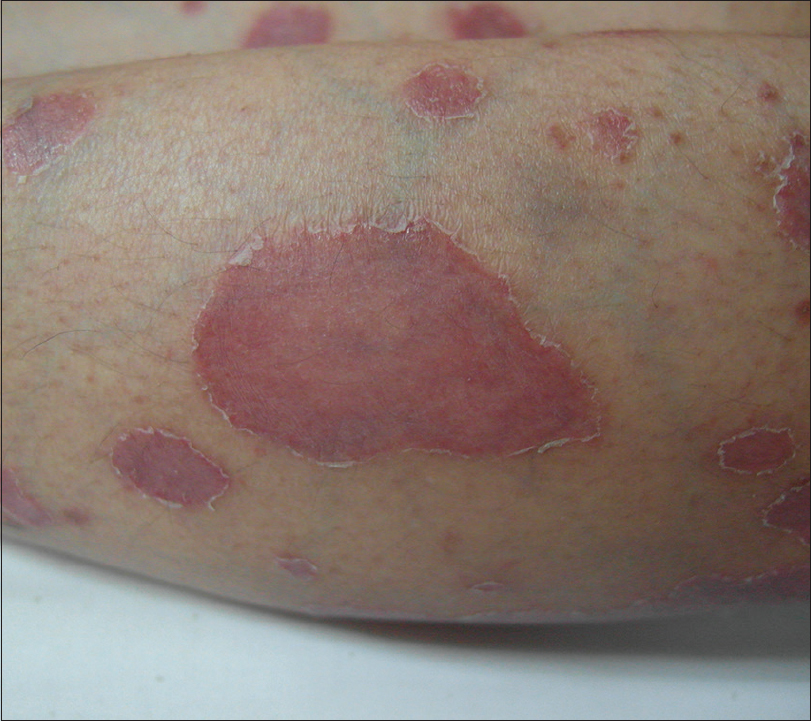 |
| Figure 1: Multiple well-demarcated, erythematous plaques with scales which are predominantly at the edges. First presentation of psoriasis at 37 years old. |
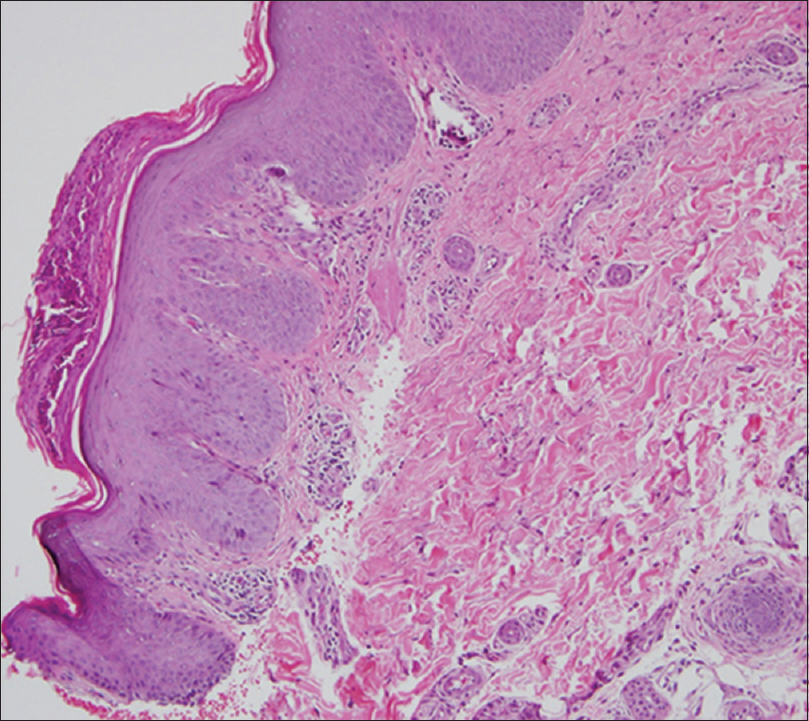 |
| Figure 2: Psoriasiform epidermal hyperplasia, hyperkeratosis, hypogranulosis, elongation of dermal papillae with thin suprapapillary plates and dilated superficial blood vessels consistent with psoriasis.(H and E, ×10) |
Management of patients with concomitant SLE and psoriasis is challenging. The drugs used must be carefully evaluated to avoid exacerbation of the other disease and to minimize morbidities. Antimalarial agents commonly used for musculoskeletal and cutaneous manifestations of SLE may precipitate psoriasis flare. Psoriasis frequently rebounds when systemic steroids are withdrawn. Methotrexate, ciclosporin, and azathioprine are effective for both psoriasis and SLE. Acitretin is beneficial for psoriasis and has been used in SLE despite its photosensitizing potential. Phototherapy is effective in psoriasis; however, ultraviolet radiation is a well-known SLE exacerbating factor.
Ustekinumab has been used in the treatment of SLE following advances in research into the roles of IL-17 and IL-23 in its pathophysiology. A few cases of concomitant psoriasis, psoriatic arthropathy, and SLE have been successfully treated with ustekinumab.[1],[2],[3] Lupus nephritis was present in one case,[1] while both thrombocytopenia and autoimmune hemolytic anemia were present in another case.[2] Cutaneous lupus including subacute cutaneous lupus erythematosus (SCLE), discoid cutaneous lupus (DLE), and chronic cutaneous lupus erythematosus (CCLE) has responded well to ustekinumab in cases with and without concomitant psoriasis.[2],[3] A randomized, placebo-controlled, phase II trial of 102 patients with active SLE reported that ustekinumab, when added to standard therapy resulted in significant SLE response index (SRI-4) improvement, achieved by 60% of patients compared with 31% in placebo and significant changes in SLEDAI-2K at week 24.[4] There was no difference in the BILAG-Based Composite Lupus Assessment (BICLA).[4]
The use of ustekinumab in SLE is promising. It would be a very useful treatment option in patients with coexisting SLE and psoriasis; however, contrary to the favorable reports on the efficacy of ustekinumab in CLE and SLE, we observed severe flares in our patient during ustekinumab therapy. The characteristics of patients who will most likely respond to treatment need to be delineated further. A single drug that is able to treat both psoriasis and SLE is desirable to minimize adverse reactions and cost.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Chyuan IT, Tsai TH, Chang TH, Wu CS. Ustekinumab treatment in a patient with psoriasis and SLE. Lupus 2015;24:650-1.
[Google Scholar]
|
| 2. |
Kugasia A, Annem C, Jaiswal P. Successful use of ustekinumab in a patient with psoriasis, psoriatic arthropathy and SLE: A case report and review of literature. Biomed J Sci Tech Res 2017;1:7.
[Google Scholar]
|
| 3. |
Varada S, Gottlieb AB, Merola JF, Saraiya AR, Tintle SJ. Treatment of coexistent psoriasis and lupus erythematosus. J Am Acad Dermatol 2015;72:253-60.
[Google Scholar]
|
| 4. |
Van Vollenhoven R, Hahn B, Tsokos G, Wagner C, Lipsky P, Hsu B, et al. S7A: 8 Efficacy and safety of ustekinumab, an interleukin 12/23 inhibitor, in patients with active SLE: Results of a phase 2, randomized placebo-controlled study. BMJ 2018;5:1:28-9.
[Google Scholar]
|
Fulltext Views
5,329
PDF downloads
2,599





