Translate this page into:
Expression of pigment epithelium-derived factor in psoriasis, verrucae, squamous cell carcinoma and normal skin: An immunohistochemical study
2 Department of Dermatology, Venereology and Andrology, Faculty of Medicine, Benha University, Benha, Egypt
Correspondence Address:
Essam M Akl
Department of Dermatology, Venereology and Andrology, Faculty of Medicine, Benha University, Benha
Egypt
| How to cite this article: Elbalshy AM, El-Refaie AM, Akl EM. Expression of pigment epithelium-derived factor in psoriasis, verrucae, squamous cell carcinoma and normal skin: An immunohistochemical study. Indian J Dermatol Venereol Leprol 2020;86:469 |
Abstract
Background: Preservation of homeostasis status in the skin needs an equilibrium of keratinocyte proliferation, differentiation, necrosis and apoptosis. Disturbance of these regulatory mechanisms may lead to keratinocyte neoplastic and hyperproliferative diseases. Pigment epithelium-derived factor is a glycoprotein that is endogenously produced in different tissues and has a variety of biological effects in different diseases.
Objective: To evaluate the keratinocyte expression of pigment epithelium-derived factor in normal skin and three epidermal hyperproliferative diseases, namely, psoriasis, verrucae and squamous cell carcinoma.
Methods: This study included skin biopsy samples from 80 participants who were divided into four equal groups; each containing 20 samples. The first group included skin biopsies from normal skin, the second group from psoriatic lesions, the third group from verruca vulgaris and the fourth group from squamous cell carcinoma. All tissue samples were stained with hematoxylin and eosin stain and later immunohistochemically for pigment epithelium-derived factor expression.
Results: Scores of pigment epithelium-derived factor expression were lower in squamous cell carcinoma and verruca and psoriasis than normal skin with a significant difference (P = 0.04). In addition, the pattern of pigment epithelium-derived factor expression was mainly cytoplasmic in normal skin with a significant difference with that seen in psoriasis, squamous cell carcinoma and verruca vulgaris (P = 0.001).
Conclusion: Pigment epithelium-derived factor may play a role in keratinocyte differentiation.
Introduction
The epidermis is characterized by a self-renewing ability which is maintained by the delicate regulation of keratinocyte proliferation, migration, differentiation, necrosis and apoptosis.[1] A precise balance is needed to control cellular proliferation. This balance is critical, as the excess proliferation leads to disease process while excess cell loss can lead to ulceration.[2]
The epidermis and hair follicles constantly renew, thanks to keratinocyte stem cells located in the basal layer of the epidermis and in the bulge area.[3] Keratinocyte stem cells are long-lived residents in the epidermis, do not protect their genome by asymmetric chromosome segregation and are highly resistant to apoptosis.[4] It is thus likely that they accumulate several oncogenic mutations and induce skin cancer formation. It has been shown that the same cell subset is characterized by high clonogenic potential and by great resistance to apoptosis.[5] Psoriasis, verruca and squamous cell carcinoma are dermatological diseases characterized by both hyperproliferation and neoangiogenesis.[6]
Pigment epithelium-derived factor is a neurotrophic factor and is known to have antiangiogenesis effect.[7]
Aim of the work
This study aimed to evaluate the keratinocyte expression of pigment epithelium-derived factor in normal skin and three epidermal hyperproliferative disorders characterized by both cellular hyperproliferation and new angiogenesis: plaque psoriasis, verruca vulgaris and squamous cell carcinoma.
Methods
Ethical approval
Approval of the Research Ethical Committee in the Faculty of Medicine, Benha University, Egypt was obtained before starting this study. All patients and control persons signed written informed consent. None of the participants were subjected to any harmful effect and their personal data were kept confidential.
Type of the study
This was a retrospective case-control comparative study.
This study was conducted from January 2017 to January 2018 and included skin tissue samples from 80 individuals, subdivided into four equal groups, each containing 20 skin biopsies from plaque psoriatic lesions, verrucae vulgaris, squamous cell carcinoma and normal skin. Normal skin samples were obtained from surgical pathology specimens choosing a non-sun-exposed site. The squamous cell carcinoma tissue samples used in this study were obtained after surgical excision at the surgery Department in Benha University Hospitals.
The psoriatic patients were instructed to stop topical or systemic treatment for 1 month before skin biopsy. Tissue samples from psoriatic patients were taken using a 4 mm sterile punch while verruca vulgaris biopsies were taken with surgical excision.
Histological evaluation
Tissue samples were formalin-fixed, paraffin-embedded, they were divided into two parts. The first part was stained with hematoxylin and eosin for confirmation of clinical diagnosis and grading in the case of squamous cell carcinoma while the immunohistochemical evaluation was done using the second part.
Immunostaining procedure
The immunohistochemical staining was done using the streptavidin-biotin peroxidase technique.[8] For immunostaining, 4 μm sections were dewaxed in xylene, later, rehydration in descending concentrations of ethanol was followed. Blockage of endogenous peroxidases (by incubation in 0.3% H2O2 for 30 min) was tracked by microwave treatment (15 min in 10 mmol/L sodium citrate buffer pH 6.0) for antigen retrieval. Slides were then incubated for 30 min at 25°C with mouse IgG2b monoclonal antihuman antibody to pigment epithelium-derived factor (R and D Systems, Boston, MA, USA) at 1: 80 dilution. Using an available commercial kit (Biogenex, San Ramon, CA, USA) and according to the manufacturer's instruction, specific binding was identified. Sections were rinsed in phosphate buffer saline after each step. Phosphate buffered saline was used in control specimens, instead of the primary antibody.
Interpretation of immunostaining slides
Slides were examined by an independent pathologist blinded to the participants' clinical data. Examination of negative and positive control slides was performed first to rule out nonspecific staining and to judge the effectiveness of the technique and the reagents, respectively. Examination and evaluation of pigment epithelium-derived factor staining for both patterns and scores of expression were done.
The pattern of pigment epithelium-derived factor immunoreactivity was observed as cytoplasmic and/or membranous brown staining. Immunoreactivity for pigment epithelium-derived factor was evaluated semiquantitatively. In this semiquantitative grading method, the overall score of staining was calculated by multiplying the percentage of positive cells stained in 10 microscopic fields and the grade of pigment epithelium-derived factor expression was scored into three grades based on the percentage of epidermal expression as follows: low, moderate and high (1–10%, 11–50%, >50%, respectively).
Statistical analysis
The data were analyzed by mean ± SD, number and percentage of expression in each group. As both variables in this study are of a qualitative type, their analysis needed a chi-square test or its correction (the Fischer exact test). Chi-square test was used in comparing tow outcomes while Fischer exact test is used with three outcomes. A P value of less than 0.05 was considered significant. Statistical analysis was achieved using the statistical package for the social sciences program (v19; SPSS Inc., Chicago, IL, USA) for Microsoft Windows 7®.
Results
Skin biopsy samples from 80 subjects with a mean age of 45.16 ± 14.86 years were used in this study. Their data is summarized in [Table - 1].
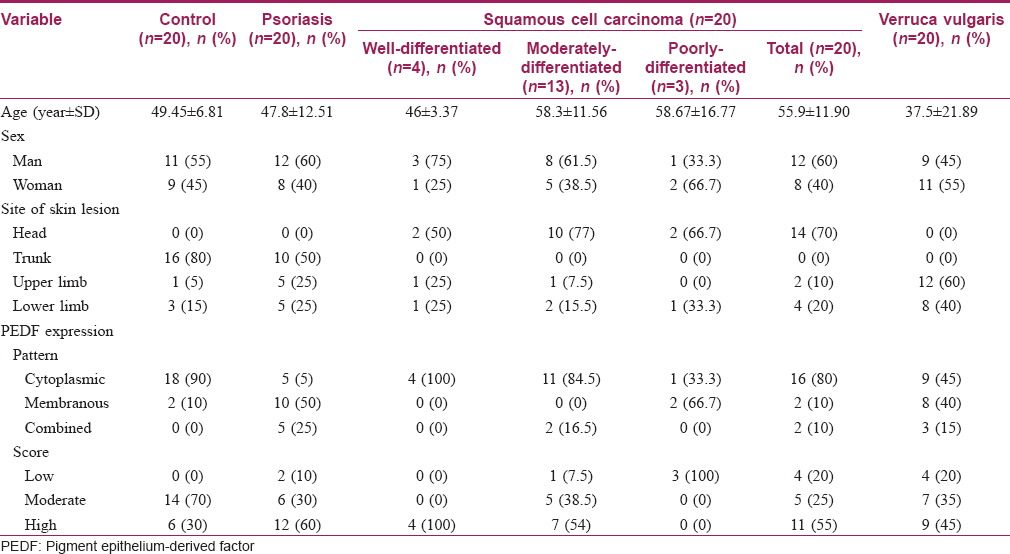
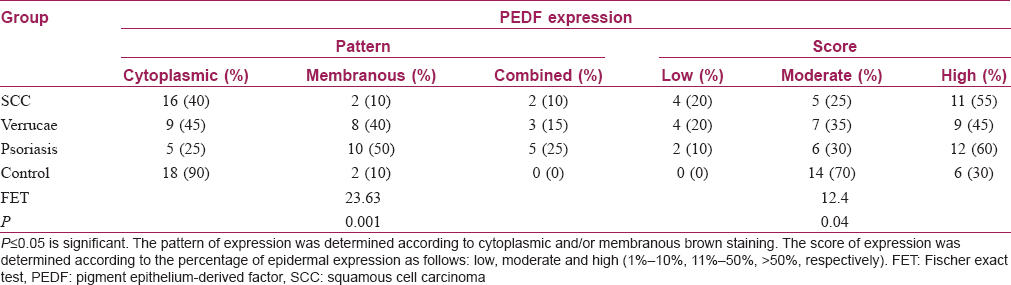
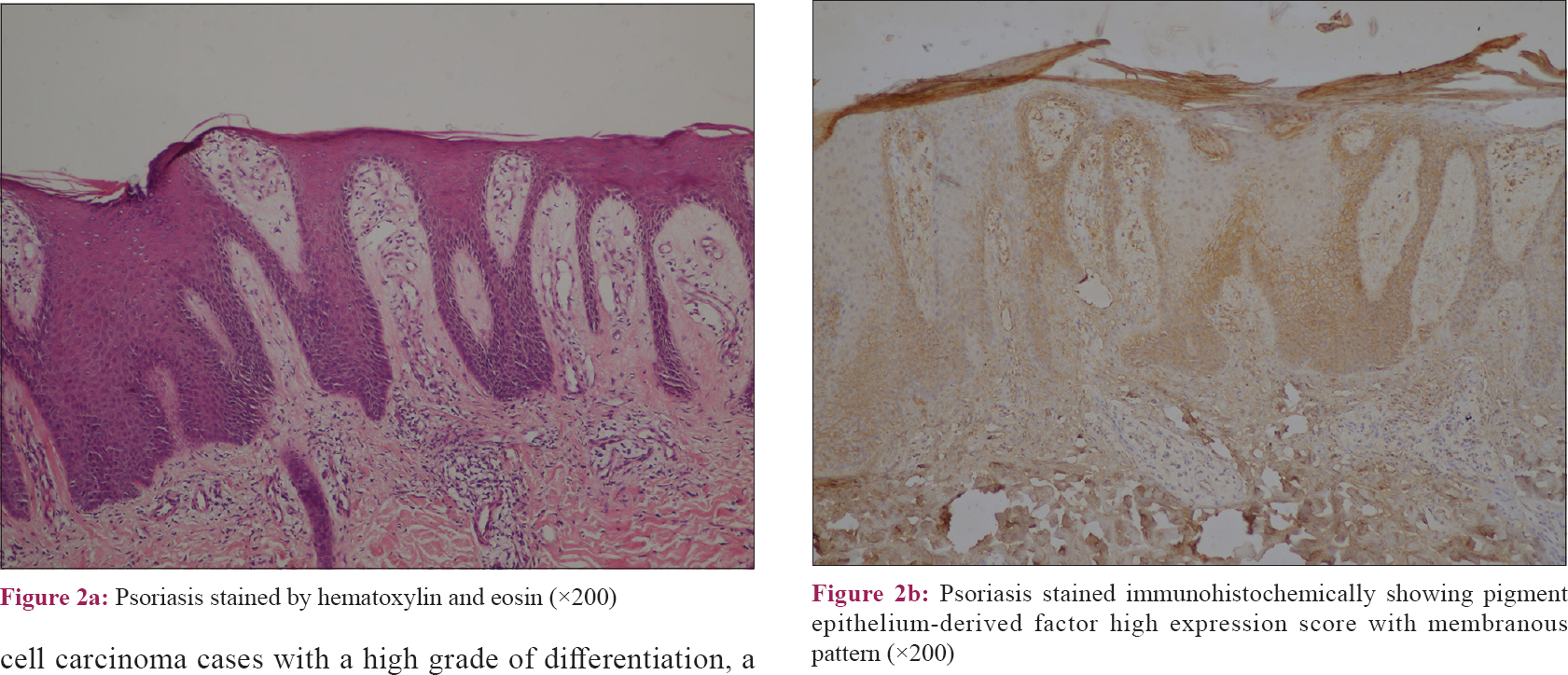
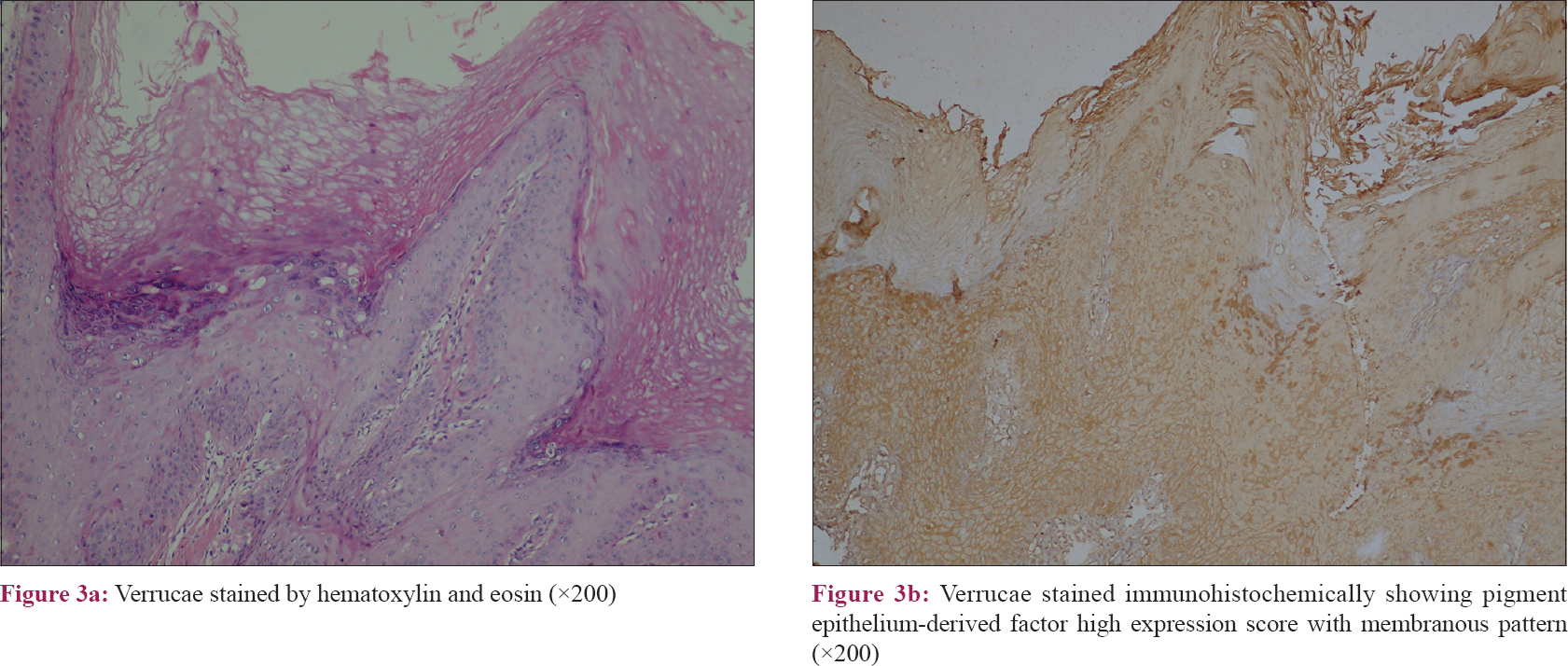
The results of this study revealed that the pattern of pigment epithelium-derived factor expression was mainly cytoplasmic in control samples with a significant difference than other included diseases (P value = 0.001). Moreover, there was a significant difference regarding the expression score of pigment epithelium-derived factor between the studied groups (P value = 0.04) [Table - 2]. Nevertheless, squamous cell carcinoma and verrucae vulgaris showed a lower score of expression followed by psoriasis while there was no low score observed in the control group [Table - 2] and [Figure - 1], [Figure - 2], [Figure - 3], [Figure - 4].
 |
| Figure 1: |
 |
| Figure 2: |
 |
| Figure 3: |
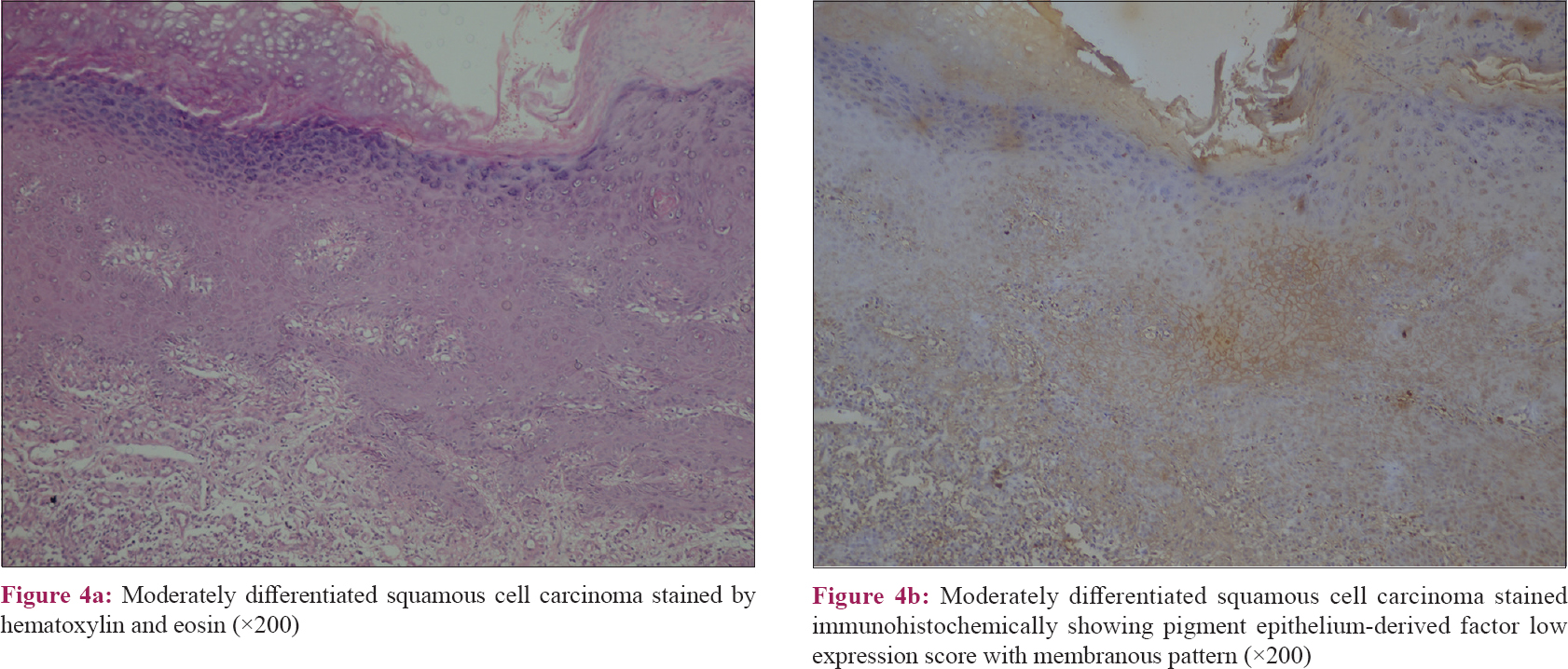 |
| Figure 4: |
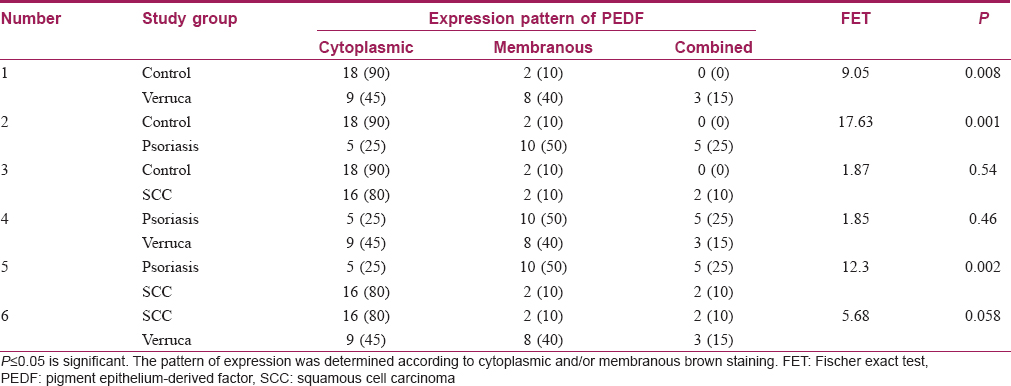
By comparing the studied groups, results showed significant differences regarding the pattern of pigment epithelium-derived factor expression between control and both verruca vulgaris and psoriasis (P value= 0.008, 0.001, respectively). In addition, there was a significant difference found between psoriasis and squamous cell carcinoma (P value= 0.002). However, there were no significant differences between verruca vulgaris and both psoriasis and squamous cell carcinoma [P value= 0.46, 0.058, respectively, [Table - 3].
The results of this study showed that there was no significant difference between all SCC cases and control regarding the expression pattern of pigment epithelium-derived factor (P value= 0.06) [Table - 3]. However, comparing the subtypes of squamous cell carcinoma included in this study and control, there was a significant difference (P value= 0.036) [Table - 4].
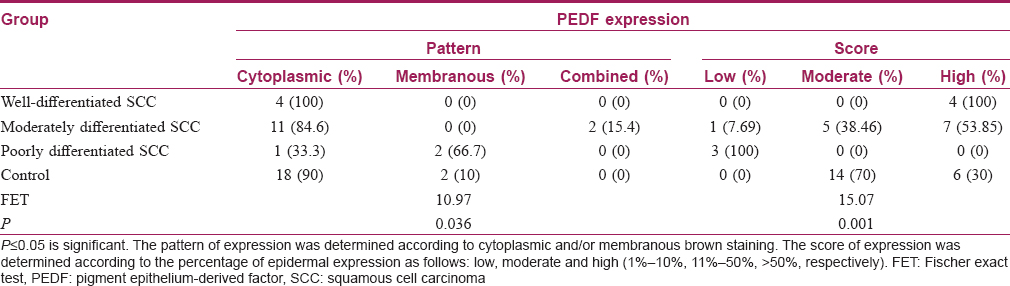
The results of this study showed that the expression score of pigment epithelium-derived factor was high in all squamous cell carcinoma cases with a high grade of differentiation, a higher score in seven cases with a moderately differentiated and low score in poorly differentiated squamous cell carcinoma cases. Comparing between different grades of the squamous cell carcinoma and control group, there was a significant difference regarding the expression score of pigment epithelium-derived factor (P value= 0.036) [Table - 4].
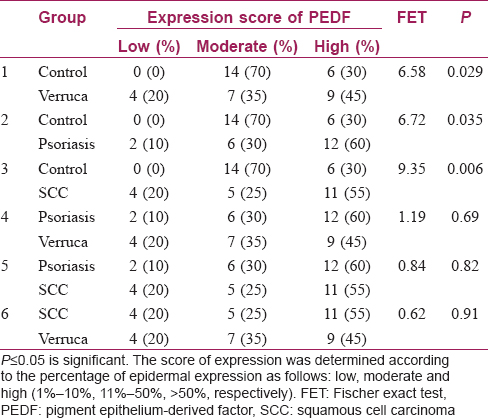
There were significant differences between the control and verruca vulgaris, psoriasis and squamous cell carcinoma regarding expression scores of pigment epithelium-derived factor (P value= 0.029, 0.035, 0.006, respectively). However, there were no significant differences between psoriasis and both verruca vulgaris and squamous cell carcinoma (P value= 0.69, 0.82, respectively). In addition, there was no significant difference between the verruca vulgaris and SCC (P value = 0.91) [Table - 5].
Discussion
A common feature of psoriasis, skin cancer and verrucae is the expression of antiapoptotic markers with different patterns of staining.[9]
Pigment epithelium-derived factor is a glycoprotein that is endogenously produced.[10] As a noninhibitory member of the serine protease inhibitor gene family[11] and a protein secreted by nearly all normal cells, bioactivities for pigment epithelium-derived factor have been expanded to include tumor suppression, cell growth and metabolism.[12] pigment epithelium-derived factor is a multifunctional, pleiotropic protein which is known for its antiangiogenic, antiproliferative, anti-inflammatory, antioxidant, neuroprotective and antithrombotic effects.[13]
Pigment epithelium-derived factor expression pattern in this study was mainly cytoplasmic in cases of low proliferation rate and highly differentiated keratinocyte (normal skin and well-differentiated squamous cell carcinoma). In contrast, pigment epithelium-derived factor expression pattern was mainly membranous in cases of high proliferation rate (psoriasis and poorly-differentiated squamous cell carcinoma). Although the score of expression differed, this was in accordance with Bowen et al., who stated that the proliferative index of psoriasis is more than squamous cell carcinoma and verruca.[14] Pigment epithelium-derived factor is strongly immunolocalized in the nucleus of many mammalian cells, suggesting that pigment epithelium-derived factor could migrate to the nuclear compartment to perform a specific function such as regulation of cell cycle.[15] The presence of cytoplasmic pigment epithelium-derived factor may control the cell cycle and prevent proliferation through interaction with the nuclear receptor corepressor 1[16] that can repress transcription factors as activator protein (AP)-1,[17] nuclear factor-kappa β (NF-κβ)[18] and retinoid receptors (retinoic acid receptor, retinoid X receptor),[19] which have roles in cellular inflammation[20] proliferation, differentiation and apoptosis.[21]
The membranous expression of pigment epithelium-derived factor may be required to exert its antiangiogenic potential which is counteracted by other angiogenesis promoting factors, upregulated in hyperproliferative keratinocyte disorders.[22] Pigment epithelium-derived factor appears to be a selective inhibitor of angiogenesis, sparing the preexisting vasculature and targeting de novo vasculature growth only.[23] The pathological angiogenesis occurs in conditions as tumor growth and chronic inflammation such as psoriasis, hence, pigment epithelium-derived factor is expected to have a role in these diseases.
These pleiotropic effects of pigment epithelium-derived factor are due to different target receptors and each has a distinctive function. The first receptor is pigment epithelium-derived factor receptor A which is located intracellularly and responsible for cell differentiation, whereas the other receptor is pigment epithelium-derived factor receptor A which is detected in plasma membranes and involved in cellular adhesion, apoptosis and angiogenesis.[24]
Pigment epithelium-derived factor expression score in this study was correlated with keratinocyte differentiation. Our results were in accordance with previous studies regarding verrucae[25] and squamous cell carcinoma.[26]
Regarding psoriasis, a study by Abe et al. showed that the pigment epithelium-derived factor expression in uninvolved lesions was observed to be much higher than that in psoriatic lesions and topical application of peptide mimetics of pigment epithelium-derived factor led to reducing both epidermal thickness and angiogenesis in a mouse model of psoriatic disease.[27] Keratinocytes can produce pigment epithelium-derived factor with its functions and wide pattern of expression; pigment epithelium-derived factor has been suggested to be a factor that promotes the return to tissue homeostasis following a pathologic insult.[28]
Furthermore, pigment epithelium-derived factor can be used as a marker of both cellular differentiation and proliferation in cases of malignancy.[29]In vitro, the addition of pigment epithelium-derived factor in cellular culture can inhibit proliferation and promote apoptosis of tumor cells.[30] While in vivo, measuring pigment epithelium-derived factor's concentration within the tissue or fluid of a cancer patient may define whether the tumor is in early or advanced stages of tumorigenesis.[31]
Conclusion
Pigment epithelium-derived factor plays a role in keratinocyte differentiation, proliferation, skin angiogenesis and inflammation-related disease processes. The expression pattern of pigment epithelium-derived factor may be used as a marker of keratinocyte differentiation.
Recommendation
Large-scale studies to evaluate the role of pigment epithelium-derived factor as a targeted therapy in skin proliferative lesions are recommended and topical application of pigment epithelium-derived factor may be used in hyperproliferative skin diseases.
Limitations
Poorly differentiated squamous cell carcinoma and squamous cell carcinoma in situ were not included in this study as only four samples of poorly differentiated squamous cell carcinoma were available.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Shirakata Y. Regulation of epidermal keratinocytes by growth factors. J Dermatol Sci 2010;59:73-80.
[Google Scholar]
|
| 2. |
Doupé DP, Jones PH. Interfollicular epidermal homeostasis: Dicing with differentiation. Exp Dermatol 2012;21:249-53.
[Google Scholar]
|
| 3. |
Blanpain C, Fuchs E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 2009;10:207-17.
[Google Scholar]
|
| 4. |
Sotiropoulou PA, Candi A, Blanpain C. The majority of multipotent epidermal stem cells do not protect their genome by asymmetrical chromosome segregation. Stem Cells 2008;26:2964-73.
[Google Scholar]
|
| 5. |
Harper LJ, Costea DE, Gammon L, Fazil B, Biddle A, Mackenzie IC. Normal and malignant epithelial cells with stem-like properties have an extended G2 cell cycle phase that is associated with apoptotic resistance. BMC Cancer 2010;10:166.
[Google Scholar]
|
| 6. |
Richarz NA, Boada A, Carrascosa JM. Angiogenesis in dermatology – Insights of molecular mechanisms and latest developments. Actas Dermosifiliogr 2017;108:515-23.
[Google Scholar]
|
| 7. |
Craword SE, Fitchev P, Veliceasa D, Volpert OV. The many facets of PEDF in drug discovery and disease: A diamond in the rough or split personality disorder? Expert Opin Drug Discov 2013;8:769-92.
[Google Scholar]
|
| 8. |
Li CM, Li W, Man XY, Liu ZG, Zheng M. Expression of pigment epithelium-derived factor in human cutaneous appendages. Clin Exp Dermatol 2013;38:652-8.
[Google Scholar]
|
| 9. |
Raj D, Brash DE, Grossman D. Keratinocyte apoptosis in epidermal development and disease. J Invest Dermatol 2006;126:243-57.
[Google Scholar]
|
| 10. |
Becerra SP. Focus on Molecules: Pigment epithelium-derived factor (PEDF). Exp Eye Res 2006;82:739-40.
[Google Scholar]
|
| 11. |
Becerra SP, Sagasti A, Spinella P, Notario V. Pigment epithelium-derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. J Biol Chem 1995;270:25992-9.
[Google Scholar]
|
| 12. |
Fitchev P, Chung C, Plunkett BA, Brendler CB, Crawford SE. PEDF and stem cells: niche vs. nurture. Curr Drug Deliv 2014;11:552-60.
[Google Scholar]
|
| 13. |
Rychli K, Huber K, Wojta J. Pigment epithelium-derived factor (PEDF) as a therapeutic target in cardiovascular disease. Expert Opin Ther Targets 2009;13:1295-302.
[Google Scholar]
|
| 14. |
Bowen AR, Hanks AN, Murphy KJ, Florell SR, Grossman D. Proliferation, apoptosis, and survivin expression in keratinocytic neoplasms and hyperplasias. Am J Dermatopathol 2004;26:177-81.
[Google Scholar]
|
| 15. |
Kawaguchi T, Yamagishi SI, Sata M. Structure-function relationships of PEDF. Curr Mol Med 2010;10:302-11.
[Google Scholar]
|
| 16. |
Doyon G, St-Jean S, Darsigny M, Asselin C, Boudreau F. Nuclear receptor co-repressor is required to maintain proliferation of normal intestinal epithelial cells in culture and down-modulates the expression of pigment epithelium-derived factor. J Biol Chem 2009;284:25220-9.
[Google Scholar]
|
| 17. |
Ogawa S, Lozach J, Jepsen K, Sawka-Verhelle D, Perissi V, Sasik R, et al. A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. Proc Natl Acad Sci U S A 2004;101:14461-6.
[Google Scholar]
|
| 18. |
Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell 2004;116:511-26.
[Google Scholar]
|
| 19. |
Weston AD, Blumberg B, Underhill TM. Active repression by unliganded retinoid receptors in development: Less is sometimes more. J Cell Biol 2003;161:223-8.
[Google Scholar]
|
| 20. |
Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev 2005;208:126-40.
[Google Scholar]
|
| 21. |
Li M, Sun Y, Guan X, Shu X, Li C. Advanced progress on the relationship between RA and its receptors and malignant tumors. Crit Rev Oncol Hematol 2014;91:271-82.
[Google Scholar]
|
| 22. |
Baba H, Yonemitsu Y, Nakano T, Onimaru M, Miyazaki M, Ikeda Y, et al. Cytoplasmic expression and extracellular deposition of an antiangiogenic factor, pigment epithelium-derived factor, in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2005;25:1938-44.
[Google Scholar]
|
| 23. |
Yamagishi SI, Matsui T. Pigment epithelium-derived factor: A novel therapeutic target for cardiometabolic diseases and related complications. Curr Med Chem 2018;25:1480-500.
[Google Scholar]
|
| 24. |
Belkacemi L, Zhang S ×. Anti-tumor effects of pigment epithelium-derived factor (PEDF): Implication for cancer therapy. A mini-review. J Exp Clin Cancer Res 2016;35:4.
[Google Scholar]
|
| 25. |
Dong YH, Li ZQ, Sun Y, Zhuang L, Wang YK, Sun Q. Downregulation of pigment epithelium-derived factor in condyloma acuminatum. J Int Med Res 2013;41:365-70.
[Google Scholar]
|
| 26. |
Miyagishi D, Ohno-Matsui K, Amagasa T, Morita I. Regulation of the expression of pigment epithelium-derived factor, an anti-angiogenic factor in human oral squamous cell carcinoma cell lines. Cancer Lett 2003;196:77-85.
[Google Scholar]
|
| 27. |
Abe R, Yamagishi S, Fujita Y, Hoshina D, Sasaki M, Nakamura K, et al. Topical application of anti-angiogenic peptides based on pigment epithelium-derived factor can improve psoriasis. J Dermatol Sci 2010;57:183-91.
[Google Scholar]
|
| 28. |
Chen L, DiPietro LA. Production and function of pigment epithelium-derived factor in isolated skin keratinocytes. Exp Dermatol 2014;23:436-8.
[Google Scholar]
|
| 29. |
Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: A multi-functional serpin family protein. J Cell Biochem 2009;106:769-75.
[Google Scholar]
|
| 30. |
Becerra SP, Notario V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nat Rev Cancer 2013;13:258-71.
[Google Scholar]
|
| 31. |
Manalo KB, Choong PF, Becerra SP, Dass CR. Pigment epithelium-derived factor as an anticancer drug and new treatment methods following the discovery of its receptors: a patent perspective. Expert Opin Ther Pat 2011;21:121-30.
[Google Scholar]
|
Fulltext Views
3,402
PDF downloads
3,323





