Translate this page into:
Extensive disseminated cysticercosis
2 Department of Pathology, Military Hospital, Lucknow Cantonment, Lucknow, Uttar Pradesh, India
3 Department of Radiology, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Sujay Khandpur
Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi - 110 029
India
| How to cite this article: Khandpur S, Kothiwala SK, Basnet B, Nangia R, Venkatesh H A, Sharma R. Extensive disseminated cysticercosis. Indian J Dermatol Venereol Leprol 2014;80:137-140 |
Abstract
Cysticercosis, especially neurocysticercosis, is a major public health problem in India. We report an unusual case of disseminated cysticercosis with extensive infiltration of the skin, central nervous system, skeletal muscles, eye, lung, and heart. A patient with extensive cutaneous cysticercosis must be thoroughly investigated for widespread internal organ involvement.Introduction
Cysticercosis caused by Cysticercus cellulosae, a larval stage of the tapeworm Taenia solium, is a major public health problem, especially in the developing world. [1] The commonest form is neurocysticercosis (NCC) and this is the single most common cause of epilepsy in developing countries. Disseminated cysticercosis occurring as a result of widespread invasion of the larval form into various tissues is a rare manifestation of cysticercosis. Fewer than 60 cases of disseminated infection have been reported worldwide. [1] We report a case of disseminated cysticercosis in an immunocompetent individual with extensive involvement of the skin, skeletal muscles, brain, vertebral bodies, eye muscles, lungs, and heart.
Case Report
A 48-year old man presented with innumerable soft to firm, deep-seated asymptomatic nodular swellings over the trunk and extremities since 7-8 years. Initially, he noticed these lesions over the shoulders and chest, with gradual involvement of extremities, back and face. He also complained of headache on and off and 6-7 episodes of seizures over the last 6 years for which he had received an antiepileptic agent for 5 years. For 3-4 years, he had noted altered behavior, irritability, and amnesia. There was no history suggestive of systemic involvement. He stated that he occasionally ate pork. There was no history of seizures or similar complaints in the family. Dermatological examination revealed multiple subcutaneous, skin colored, soft to firm and cystic, discrete, and confluent asymptomatic nodules of size 0.5 cm to 3-4 cm, distributed predominantly over the shoulders, upper chest, back, and both arms [Figure - 1]. General physical and systemic examination were within normal limits.
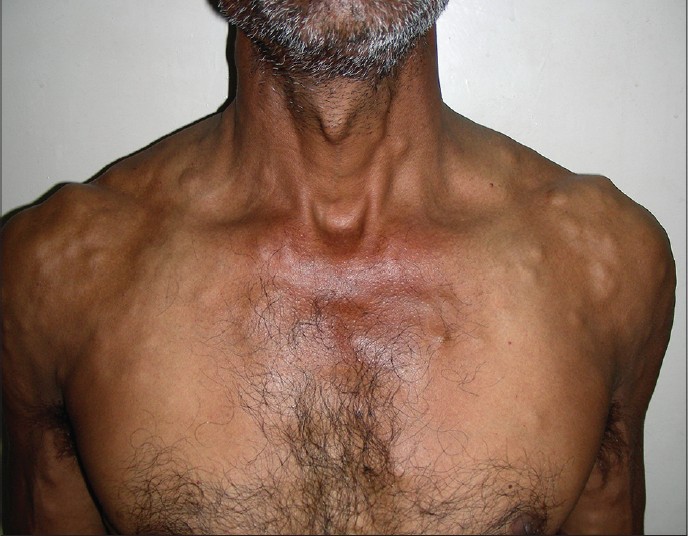 |
| Figure 1: Multiple cutaneous nodules over chest, shoulders, and arms of cutaneous cysticercosis |
Investigations including complete blood count, liver and renal function tests, urine and stool analysis, and electrocardiogram were within normal limits. Skin biopsy from a chest nodule showed the characteristic cysticercus in the deep dermis [Figure - 2]. Ultrasound of abdomen and lower chest showed multiple cysts in bilateral psoas, right crura, bilateral pectoral, and abdominal muscles. Ultrasound A scan of eyes showed cysts in the rectus muscles with 100% spikes suggestive of cysticercosis. Computed tomography (CT) scan of head revealed involvement of cerebrum, extraocular muscles, temporalis and occipito-frontalis muscles, with lesions in vesicular, colloidal, and predominantly calcified stages. Whole body magnetic resonance imaging (MRI) revealed cysticerci in bilateral cerebral and cerebellar hemispheres, spinal cord, multiple skeletal muscles, lower lobe of right lung, and T9 and T11 vertebral bodies [Figure - 3]a and b. An electroencephalogram was normal. Echocardiography showed two cysts of size 2 × 3 cm in the anterior cardiac wall. A diagnosis of disseminated cysticercosis was made.
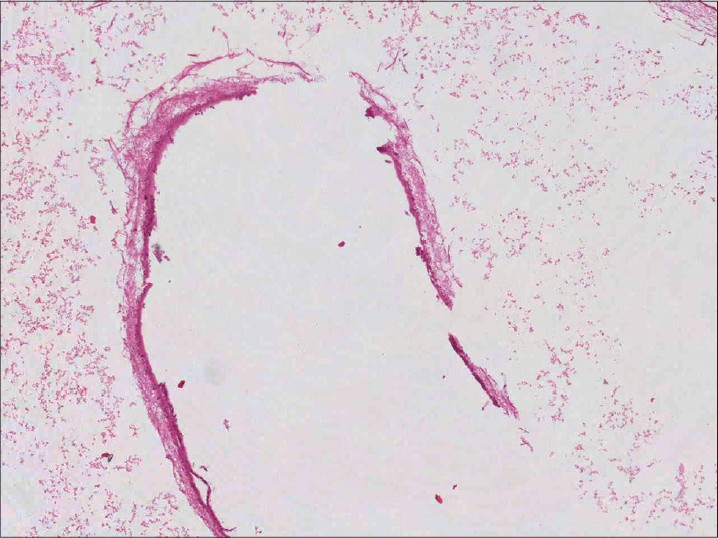 |
| Figure 2: Photomicrograph showing cysticercosis in the dermis (H and E, ×100) |
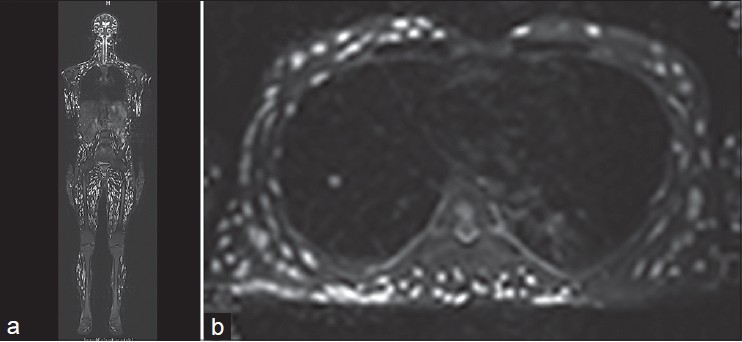 |
| Figure 3: (a and b) Coronal short TI inversion recovery whole body magnetic resonance imaging shows disseminated cysticercus lesions in skeletal muscles from head to toe, lesions are also noted in the cerebral hemisphere, spinal cord (a). Axial T2W fat suppressed image showing cysticercus lesions in the lower lobe of right lung, pleural cavity (b) |
Treatment with praziquantel carried the risk of severe and potentially fatal hypersensitivity reaction and severe inflammatory episodes following massive release of larval antigens in spite of premedication with steroids and antihistamines. In view of this, we decided not to administer praziquantel particularly since his skin nodules were asymptomatic and he had no systemic complaints. He was prescribed paracetamol for headache and carbamazepine 200 mg twice daily for prevention of seizures. We have explained the nature of the disease and its likely consequences to the patient and he continues to follow up with us.
Discussion
Human cysticercosis is a major public health problem both in resource-poor as well as developed countries. It is the single most common cause of epilepsy in most of South and Central America, India, South-east Asia, China, and sub-Saharan Africa. [1] It is caused by the dissemination of embryos of T. solium from the intestine via the hepato-portal system to various organs. Humans are the only definitive host of T. solium harboring the adult tapeworm in the intestine. Both humans and pigs are intermediate hosts and harbour T. solium, larvae in different internal organs. [2] These larval stages are rapidly destroyed by the host′s immune system in most circumstances, except for those located in immunologically privileged sites like the nervous system. Ingestion of food contaminated with T. solium eggs and faeco-oral auto-transmission in individuals harbouring the intestinal cestode are two main sources from which humans contact cysticercosis. [3] It is common in communities where people consume undercooked pork although the proportion of pork eaters among Indian patients is low, an unusual feature of the disease in our country. [3] Less than 1-2% of patients with neurocysticercosis are pork eaters and more than 95% of Indian patients are vegetarian. [4] This finding indicates generalized exposure to T. solium eggs through contaminated fruits or raw vegetables eaten as salad. Sensitivity of stool examinations is poor and only about 15% of patients harbour a tapeworm at the time of diagnosis of neurocysticercosis. [5]
The various organs invaded by cysticerci besides the brain include subcutaneous tissue, skeletal muscles, lungs, eyes, liver, and occasionally the heart. Krishnaswami [6] and Priest [7] reported cysticerci manifesting as subcutaneous nodules in association with abundant cysticerci in the muscles, heart, and brain. Disseminated cysticercosis is relatively rare and fewer than 60 cases have been previously reported. [1] Clinical manifestations of the disease depend upon the location of the cyst, cyst burden, and the host reaction.
Neurocysticercosis is the commonest form reported with the brain parenchyma being the commonest site, followed by meninges, ventricles, eye, and spinal cord. [8] The manifestations of neurocysticercosis are polymorphic; ranging from no symptom or sign to acute symptomatic seizures, headache, hydrocephalus, chronic meningitis, focal neurological deficits, psychological disorders, dementia, and ocular and spinal cysts. The solitary form of the disease (solitary cysticercus granuloma) is the commonest presentation, reported in nearly two-thirds of all patients with neurocysticercosis. Our patient had involvement of brain parenchyma with multiple lesions and presented with a history of seizures, recurrent headache, irritability and amnesia.
Intraocular cysticercosis manifests with proptosis, diplopia, and loss of vision, while the extraocular cyst resembles a slow-growing tumour or nodule with focal inflammation. Our patient had cysts in the rectus muscles but did not have any ocular complaint.
Cysts in muscles may manifest as muscular pain, weakness, or pseudohypertrophy. Rarely, due to due to heavy infection of the skeletal muscles, muscular pseudohypertrophy occurs which gives the patient a "herculean appearance." [9]
Subcutaneous cysticercosis is frequently asymptomatic but may manifest as palpable nodules as in our patient who had variable sized nodules all over the body. It has been reported that subcutaneous cysticercosis with neurocysticercosis is more common in Asia compared to America and Africa. [10] Arora et al., [11] reported a series of 33 cases of cutaneous cysticercosis, with more than one skin nodule in most, occurring most often on the trunk. and with associated neurocysticercosis in 82% of cases. Amatya and Kimula [12] described the clinical and histopathological features of cutaneous cysticercosis in 62 cases from Nepal. Most (82%) of the patients presented with solitary skin nodules, another 10% with nodules in the oral mucosa and 8% in the breast. In both series, patients were not evaluated for more extensive involvement of other organ systems as was done in our case.
Pulmonary and cardiac involvement has only rarely been described in the literature. [13],[14] Cardiac lesions can cause arrhythmias. However, our patient had involvement of the anterior wall of heart but was asymptomatic. Besides this, there was also infiltration into skeletal muscles, vertebral bodies, and thoracic cavity including right lung and pleura.
MRI is considered the best neuroimaging tool for radiological diagnosis of neurocysticercosis with the advantage of being able to differentiate the stages of the parasite (which CT fails to do). In our patient, MRI not only corroborated the clinical diagnosis but also revealed very widespread infiltration of cysticerci. Echocardiography helped us to detect involvement of the anterior wall of heart, though the electrocardiogram was normal and the patient was asymptomatic.
Management of neurocysticercosis includes antiparasitic drugs like albendazole and praziquantel, surgery, antiseizure prophylaxis, and symptomatic medication. [15] The potential efficacy of antihelminthic therapy in patients with neurocysticercosis is controversial. [16] More recently, treatment with anticysticercal drugs is favoured, which results in better resolution of single enhancing lesions, although antihelminthic therapy and steroids do not affect the development of calcification and risk of chronic epilepsy. A problem with antihelminthic therapy is that it causes aggregation of inflammatory cells around cysticerci, which often leads to transient clinical deterioration that is more deleterious in patients with a heavy parasitic load. [17] For this reason, systemic corticosteroids are given simulataneously with both albendazole and praziquantel to control the edema and intracranial hypertension that may occur following therapy. Rarely, this may be fatal in heavy infections, despite administration of corticosteroids. [18] Excision of the cyst is a treatment option for localized cutaneous cysticercosis with no associated internal organ involvement but this procedure was not possible in our case as he had hundreds of lesions all over the body. Our patient was started on symptomatic treatment for headache and prophylactic therapy for seizures since asymptomatic subcutaneous or intramuscular cysticerci do not require treatment. [15] We decided not to administer antihelminthics to avoid the risk of reaction around the extensively distributed cysts in the brain and other organs.
| 1. |
Bern C, Garcia HH, Evans C, Gonzalez AE, Verastegui M, Tsang VC, et al. Magnitude of the disease burden from neurocysticercosis in a developing country. Clin Infect Dis 1999;29:1203-9.
[Google Scholar]
|
| 2. |
Wadia NH, Singh G. Taenia Solium: A historical note. Taenia solium cysticercosis: From basic to clinical science. Wallingford: CABI Publishing; 2002. p. 157-68.
[Google Scholar]
|
| 3. |
Rajshekhar V. Epidemiology of taenia solium taeniasis/cysticercosis in India and Nepal. Southeast Asian J Trop Med Public Health 2005;35:247-51.
[Google Scholar]
|
| 4. |
Prasad KN, Prasad A, Verma A, Singh AK. Human cysticercosis and Indian scenario: A review. J Biosci 2008;33:571-82.
[Google Scholar]
|
| 5. |
Gilman RH, Del Brutto OH, Garcia HH, Martinez M. Prevalence of taeniasis among patients with neurocysticercosis is related to severity of infection. The Cysticercosis Working Group in Perú. Neurology 2000;55:1062.
[Google Scholar]
|
| 6. |
Krishnaswami CS. Case of cysticercus cellulose. Indian Med Gaz 1912;27:43-4.
[Google Scholar]
|
| 7. |
Priest R. A case of extensive somatic dissemination of cysticercus cellulose in man. Br Med J 1926;2:471-2.
[Google Scholar]
|
| 8. |
Del Brutto OH, Wadia OH, Dumas M, Cruz M, Tsang VC, Schantz PM. Proposal of diagnostic criteria for human cysticercosis and neurocysticercosis. J Neurol Sci 1996; 142:1-6.
[Google Scholar]
|
| 9. |
Garcia HH, Gilman RH. Cysticercosis. In: Oxford Textbook of Medicine. 4 th ed. New York: Oxford University Press; 2003. p. 825-6.
[Google Scholar]
|
| 10. |
Nakao M, Okamoto M, Sako Y, Yamasaki H, Nakaya K, Ito A. A phylogenetic hypothesis for the distribution of two genotypes of the pig tapeworm Taenia solium worldwide. Parasitology 2002;124:657-62.
[Google Scholar]
|
| 11. |
Arora PN, Sanchetee PC, Ramakrishnan KR, Venkataram S. Cutaneous, mucocutaneous and neurocutaneous cysticercosis. Indian J Dermatol Venereol Leprol 1990;56:115-8.
[Google Scholar]
|
| 12. |
Amatya BM, Kimula Y. Cysticercosis in Nepal: A histopathologic study of sixty-two cases. Am J Surg Pathol 1999;23:1276-9.
[Google Scholar]
|
| 13. |
Bastos AL, Marchiori E, Gasparetto EL, Andrade BH, Junior GC, Carvalho RC, et al. Pulmonary and cardiac cysticercosis: Helical CT findings. Br J Radiol 2007; 80:e58-60.
[Google Scholar]
|
| 14. |
Jain BK, Sankhe SS, Agrawal MD, Naphade PS. Disseminated cysticercosis with pulmonary and cardiac involvement. Indian J Radiol Imaging 2010;20:310-3.
[Google Scholar]
|
| 15. |
García HH, Evans CA, Nash TE, Takayanagui OM, White AC Jr, Botera D, et al. Current consensus guidelines for treatment of neurocysticercosis. Clin Microbiol Rev 2002;15:747-56.
[Google Scholar]
|
| 16. |
Rajshekhar V. Albendazole therapy in patients with solitary cerebral cysticercus granuloma. Is it effective? J Neurol Neurosurg Psychiatry 2008;79:238-9.
[Google Scholar]
|
| 17. |
Sotelo J, Escobedo F, Rodriguez-Carbajal J, Torres B, Rubio-Donnadieu F. Therapy of parenchymal brain cysticercosis with praziquantel. N Engl J Med 1984;310:1001-7.
[Google Scholar]
|
| 18. |
Wadia N, Desai S, Bhatt M. Disseminated cysticercosis. New observations, including CT scan findings and experience with treatment by praziquantel. Brain 1988; 111:597-614.
[Google Scholar]
|
Fulltext Views
11,328
PDF downloads
2,856





