Translate this page into:
Extensive milia formation in a young woman with bullous pemphigoid
2 Department of Pathology, Kasturba Medical College, Manipal University, Manipal, Karnataka, India
Correspondence Address:
Raghavendra Rao
Department of Dermatology, Kasturba Medical College, Manipal University, Manipal - 576 104, Karnataka
India
| How to cite this article: Kumudhini S, Rao R, Pai K, Shetty S, Pai S. Extensive milia formation in a young woman with bullous pemphigoid. Indian J Dermatol Venereol Leprol 2018;84:248 |
Sir,
Bullous pemphigoid is the most common subepidermal immunobullous disorder that usually affects the elderly population.[1] It is characterized clinically by the presence of tense blisters either on erythematous/urticated base or on normal-looking skin, and immunopathologically by linear deposition of immunoglobulin G and complement fraction 3 (C3) along the dermoepidermal junction. Indirect immunofluorescence microscopy on salt-split skin further helps confirm the diagnosis and typically shows immunoglobulin G staining on the epidermal side of the split (“roof-binding” pattern).[1] Autoantibodies in bullous pemphigoid are directed against the hemidesmosomal proteins – bullous pemphigoid antigen 180 and bullous pemphigoid antigen 230. It may at times be difficult to distinguish bullous pemphigoid from other subepidermal immunobullous diseases, especially epidermolysis bullosa acquisita. Younger age group, acral blistering, absence of family history, scarring and milia formation are often used as clinical criteria to differentiate epidermolysis bullosa acquisita from bullous pemphigoid.[2] Here, we report the case of a young woman with bullous pemphigoid, who uncharacteristically developed extensive milia during the recovery phase.
A 35-year-old woman presented to our department with multiple blisters all over the body since 3 months. It initially started on the chest, and then involved the scalp and the oral mucosa. Examination revealed multiple tense vesicles, bullae and erosions on the scalp, trunk and limbs; erosions were also present in the gingivae and the floor of the mouth [Figure - 1]. A provisional diagnosis of bullous pemphigoid was made; direct immunofluorescence microscopy of the perilesional skin revealed linear deposition of C3 and immunoglobulin G along the basement membrane zone. Indirect immunofluorescence study using salt-split skin showed linear staining of the basement membrane zone with immunoglobulin G on the epidermal side of the split (“roof pattern”) in 1:100 dilution, which was consistent with the clinical diagnosis of bullous pemphigoid [Figure - 2]. Enzyme-linked immunosorbent assay using recombinant bullous pemphigoid 180 and bullous pemphigoid 230 antigens showed a strong reactivity (anti-bullous pemphigoid 180 antibodies more than 200 IU/ml and anti-bullous pemphigoid 230 antibodies - 24 IU/ml). She was initially treated with oral prednisolone (1 mg/kg/day) along with supportive care. Since she continued to develop blisters at one week even after commencing this regimen, single intravenous cyclophosphamide pulse (500 mg over 3–4 h) was administered. She responded well to this regimen; at 1-month follow-up, there were no new blisters and all the existing lesions had healed with postinflammatory pigmentation. However, she complained of some new asymptomatic lesions all over the body. Examination showed multiple pearly white papules, suggestive of milia on her face and extremities [Figure - 3]. Histopathology of the representative lesion was consistent with the diagnosis of milia which showed intraepidermal cysts plugged with keratin [Figure - 4]a and [Figure - 4]b. Two more cycles of cyclophosphamide pulse were given in the subsequent months and the dose of prednisolone was tapered.
 |
| Figure 1: Tense bullae on the lower one-third of the leg |
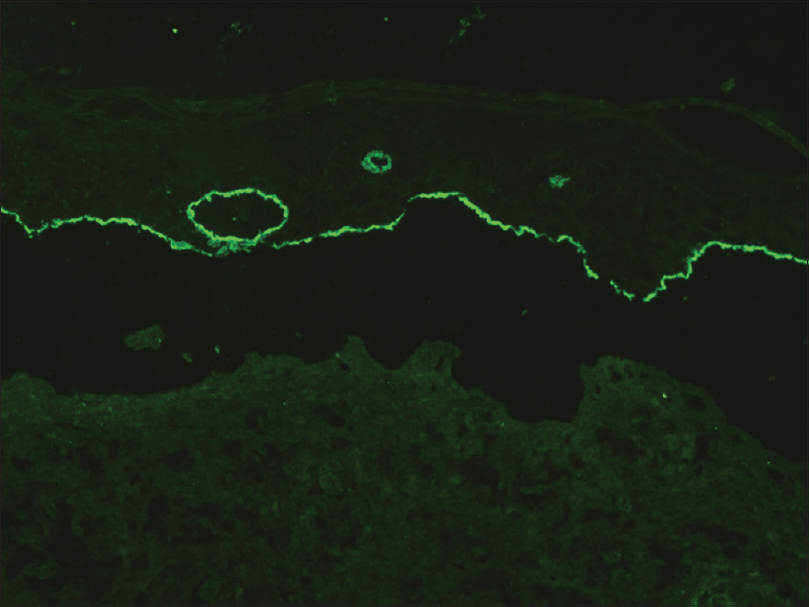 |
| Figure 2: Indirect immunofluorescence on salt-split skin showing linear deposition of immunoglobulin G on the epidermal side of the split (“roof pattern”) (FITC, ×200) |
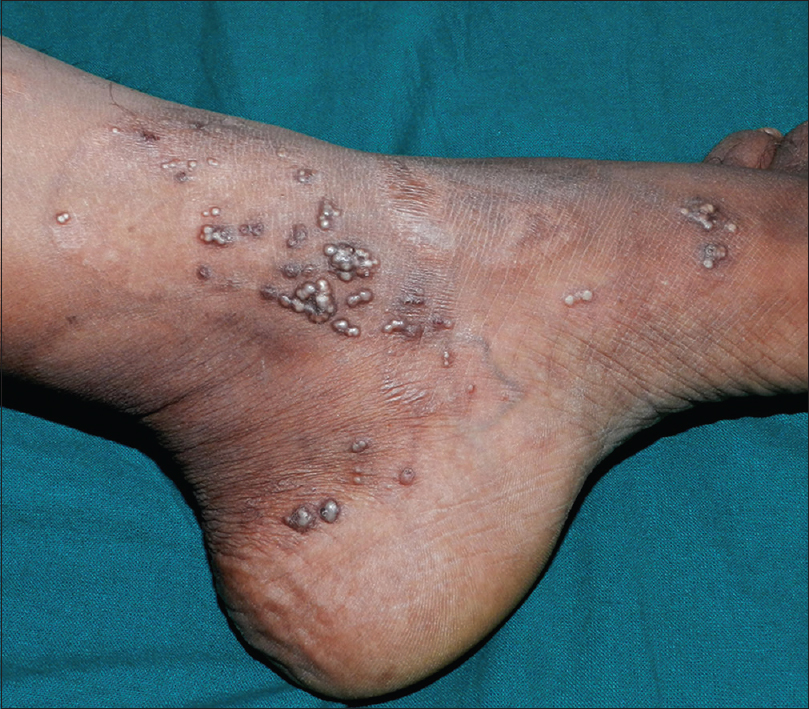 |
| Figure 3: Multiple, grouped milia on the left ankle |
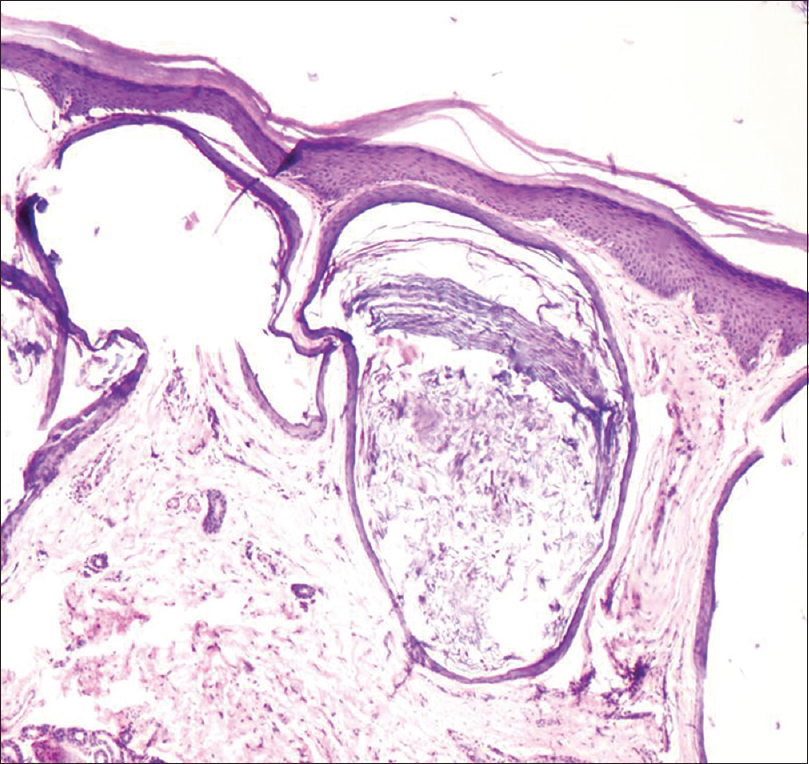 |
| Figure 4a: Multiple, small epidermoid cysts in the dermis (H and E, ×100) |
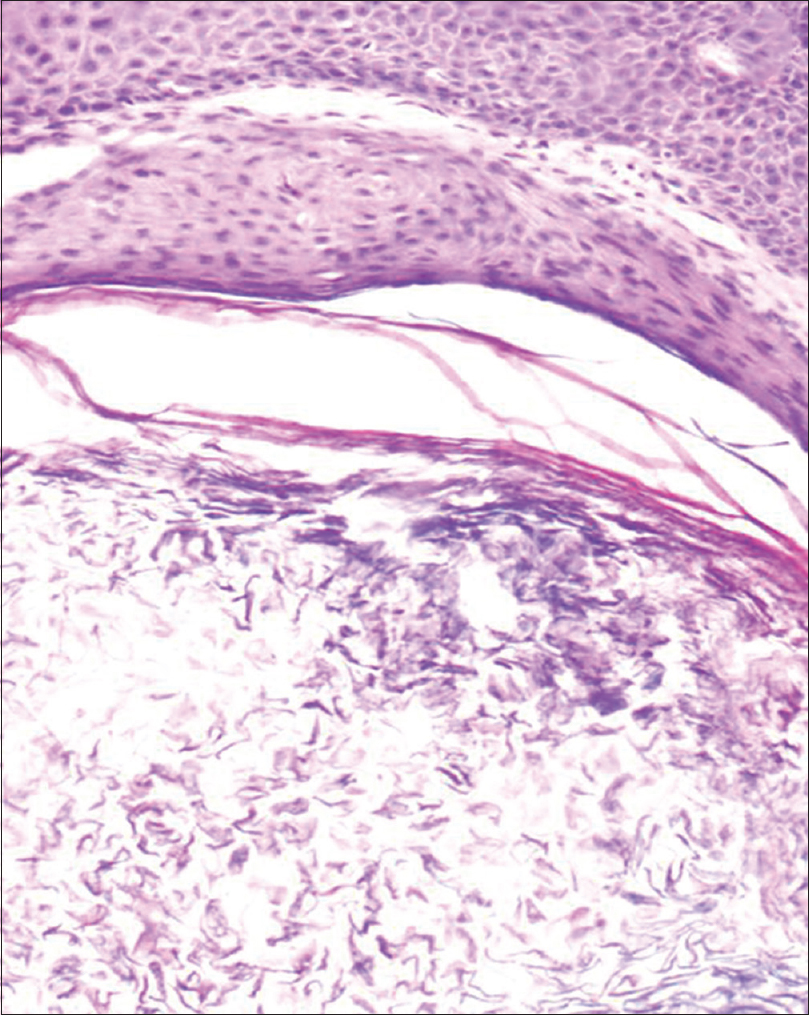 |
| Figure 4b: Cystic cavity containing laminated flakes of keratin (H and E, ×200) |
Milia are very common benign keratinous cysts of the skin, clinically seen as pearly white, dome-shaped lesions measuring 1–2 mm in diameter.[3] Two types of milia have been described; primary milia are formed from entrapped keratin and are found usually on the face whereas secondary milia are formed after trauma, burns and a variety of blistering skin conditions. Milia secondary to blisters are speculated to be produced through the regeneration process of disrupted sweat glands or hair follicles and have been reported in association with diseases such as epidermolysis bullosa (both hereditary and acquired type), porphyria cutanea tarda, mucous membrane pemphigoid, occasionally in bullous pemphigoid and the recently described p200 pemphigoid.[3],[4],[5],[6],[7] Prost et al. observed that nearly 50% of patients with epidermolysis bullosa acquisita and mucous membrane pemphigoid had milia, but none of the 15 bullous pemphigoid patients showed milia formation.[8] Although rare, milia may occasionally occur in bullous pemphigoid patients; Venning et al. and Banfield et al. reported the occurrence of milia in 38.8% (7 out of 18) and 31.1% (23 out of 74) of their bullous pemphigoid patients, respectively.[9],[10] Banfield et al. postulated a significant predisposition for milia formation in bullous pemphigoid patients with human leukocyte antigen DQ6 antigen.[10] Thus, milia formation during recovery phase may be associated with immunological predisposition. Ultrastructrally, bullous pemphigoid 180 antigen is indirectly connected to type VII collagen through laminin-332 and type IV collagen. Aberrant interaction between the hemidesmosomal proteins and the extracellular matrix components beneath the hemidesmosomes may also result in milia formation.[9] Traditionally, bullous pemphigoid is considered as a mild disease which can be treated effectively with topical and/or low-dose oral steroids.[1] Hisa et al. in 1996 reported the occurrence of milia in a 68-year-old woman; however, the diagnosis of bullous pemphigoid was not substantiated in their patient by immunological tests such as indirect immunofluorescence on salt-split skin or enzyme-linked immunosorbent assay.[4] Tsuruta et al. reported a 55-year-old woman with bullous pemphigoid who responded well to tapering doses of oral steroids; seven months later, this patient presented with multiple milia on healed bullous pemphigoid lesions.[3] Uchida et al. and Ishikawa et al. reported elderly men with bullous pemphigoid which subsequently healed with milia. Interestingly, both these patients had only bullous pemphigoid 180 antibody and did not respond satisfactorily to oral steroid therapy alone.[5],[6] Improvement was observed in their patients when adjuvant therapies such as intravenous immunoglobulin and plasma exchange were instituted. Our patient, on the other hand, had both bullous pemphigoid 180 and bullous pemphigoid 230 antibodies and required additional cyclophosphamide pulse to induce clinical remission. In summary, all the three patients (including our case) had extensive lesions and high immunological activity as indicated by bullous pemphigoid enzyme-linked immunosorbent assay. Intense inflammation along the dermoepidermal junction would have predisposed, at least partially, to the development of milia in these patients.
Bullous pemphigoid is traditionally seen in the sixth or seventh decades of life; presence of tense blisters in a young patient that heal with milia formation, as in our case, would have led erroneously to the diagnosis of epidermolysis bullosa acquisita. However, advanced immunological testing such as indirect immunofluorescence using salt-split skin and enzyme-linked immunosorbent assay using recombinant bullous pemphigoid 180 and 230 kits helped us to confirm the diagnosis of bullous pemphigoid. Postbullous milia, though uncommon, may be seen in bullous pemphigoid patients and its presence should not be used as a criterion to distinguish bullous pemphigoid from other subepidermal immunobullous diseases, especially epidermolysis bullosa acquisita. Whenever possible, the diagnosis of subepidermal immunobullous disease should be confirmed by indirect immunofluorescence on salt-split skin.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Venning VA, Taghipour K, Mohd Mustapa MF, Highet AS, Kirtschig G. British Association of Dermatologists' guidelines for the management of bullous pemphigoid 2012. Br J Dermatol 2012;167:1200-14.
[Google Scholar]
|
| 2. |
Mayuzumi M, Akiyama M, Nishie W, Ukae S, Abe M, Sawamura D, et al. Childhood epidermolysis bullosa acquisita with autoantibodies against the noncollagenous 1 and 2 domains of type VII collagen: Case report and review of the literature. Br J Dermatol 2006;155:1048-52.
[Google Scholar]
|
| 3. |
Tsuruta D, Brzezinski P, Koga H, Ohata C, Furumura M, Hashimoto T. Bullous pemphigoid with prominent milium formation. Acta Dermatovenerol Croat 2013;21:35-8.
[Google Scholar]
|
| 4. |
Hisa T, Goto Y, Taniguchi S, Nakanishi T, Kakudo K, Takigawa M. Post-bullous milia. Australas J Dermatol 1996;37:153-4.
[Google Scholar]
|
| 5. |
Uchida S, Oiso N, Koga H, Ishii N, Okahashi K, Matsuda H, et al. Refractory bullous pemphigoid leaving numerous milia during recovery. J Dermatol 2014;41:1003-5.
[Google Scholar]
|
| 6. |
Ishikawa M, Mori T, Hiraiwa T, Ohashi T, Kikuchi N, Hanami Y, et al. Multiple milia formation following refractory bullous pemphigoid. Dermatol Clin Res 2015;1:34-6.
[Google Scholar]
|
| 7. |
Dilling A, Rose C, Hashimoto T, Zillikens D, Shimanovich I. Anti-p200 pemphigoid: A novel autoimmune subepidermal blistering disease. J Dermatol 2007;34:1-8.
[Google Scholar]
|
| 8. |
Prost C, Labeille B, Chaussade V, Guillaume JC, Martin N, Dubertret L. Immunoelectron microscopy in subepidermal autoimmune bullous diseases: A prospective study of IgG and C3 bound in vivo in 32 patients. J Invest Dermatol 1987;89:567-73.
[Google Scholar]
|
| 9. |
Venning VA, Allen J, Millard PR, Wojnarowska F. The localization of the bullous pemphigoid and cicatricial pemphigoid antigens: Direct and indirect immunofluorescence of suction blisters. Br J Dermatol 1989;121:305-15.
[Google Scholar]
|
| 10. |
Banfield CC, Wojnarowska F, Allen J, George S, Venning VA, Welsh KI. The association of HLA-DQ7 with bullous pemphigoid is restricted to men. Br J Dermatol 1998;138:1085-90.
[Google Scholar]
|
Fulltext Views
3,364
PDF downloads
2,266





