Translate this page into:
Extramammary Paget disease secondary to a transitional cell carcinoma of the bladder
2 Department of Pathology, University Hospital of Guadalajara. C/Donantes de Sangre s/n, 19002, Guadalajara, Spain
Correspondence Address:
Adriana Mart�n-Fuentes
C/Donantes de Sangre s/n, 19002, Guadalajara
Spain
| How to cite this article: Mart�n-Fuentes A, S�nchez-Herreros C, Cuevas-Santos J, De Eusebio-Murillo E. Extramammary Paget disease secondary to a transitional cell carcinoma of the bladder. Indian J Dermatol Venereol Leprol 2015;81:318-320 |
Sir,
Extramammary Paget′s disease is an intraepidermal proliferation of Paget cells, which are also seen in the classic areolar-type mammary Paget′s disease. It can be divided into primary (intraepithelial adenocarcinoma arising within the epidermis) and secondary disease (intraepithelial spread of a visceral carcinoma).
A 47-year-old male presented with a 4-month history of an erythematous, erosive, and asymptomatic lesion involving the glans and urethral meatus [Figure - 1]. He had a history of in situ transitional cell carcinoma of the bladder treated with a radical cysto-prostatectomy 9 years ago. Hematoxylin-eosin stained sections of a biopsy from the plaque showed intraepithelial proliferation of pleomorphic pagetoid cells in a buckshot pattern, involving the full thickness of the epithelium [Figure - 2]a. At higher magnification, cells showed pale cytoplasm with large and round nuclei with prominent nucleoli [Figure - 2]b. The mitotic index was high. These Paget cells stained positively for epithelial mucins with colloidal iron. Immunohistochemically, the cells expressed positivity for cytokeratin (CK) 7 [Figure - 3]a, low molecular weight CK [Figure - 3]b and p53 while they were negative for S-100, CK-20 [Figure - 3]c, CDX-2 and gross cystic disease fluid protein (GCDFP-15) [Figure - 3]d. The histomorphological and immunohistochemical features, with a history of bladder carcinoma allowed us to make a diagnosis of extramammary Paget′s disease secondary to this malignancy.
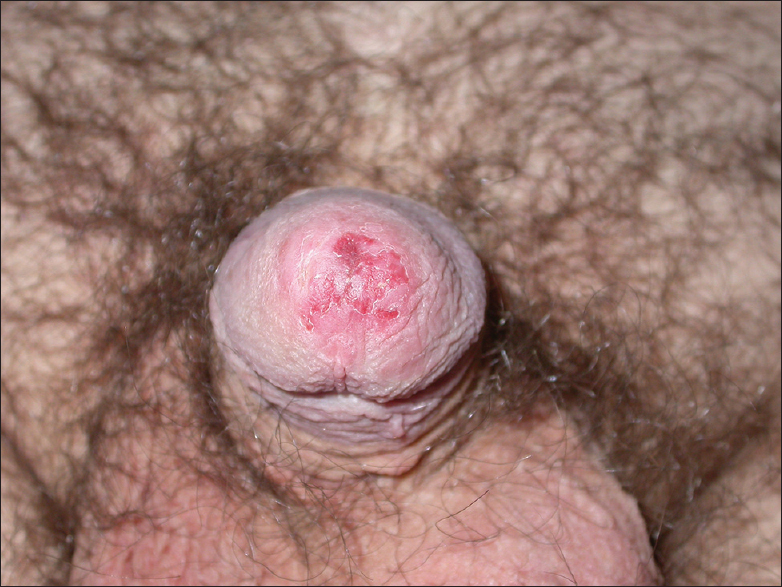 |
| Figure 1: Erythematous and erosive lesion involving glans and urethral meatus |
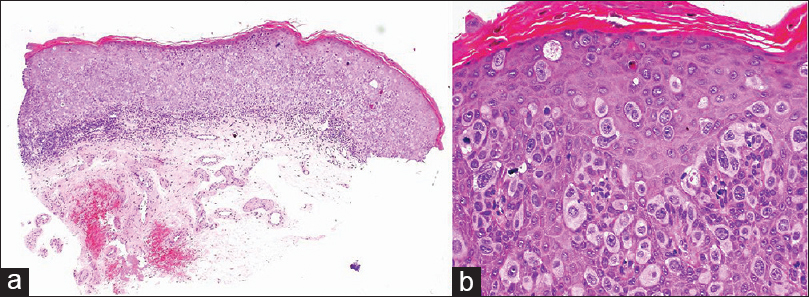 |
| Figure 2: (a) Pleomorphic pagetoid cells involving the full thickness of the epithelium in a dotted pattern. (b) Large cells with pale cytoplasm and round nuclei with prominent nucleoli (H and E, A: ×50; B: ×400) |
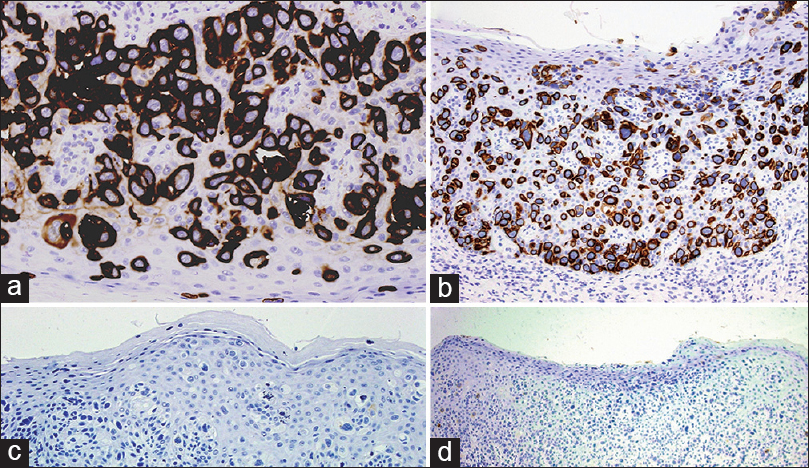 |
| Figure 3: Immunohistochemical stain. (a) CK7 (b) Low weight CK (c) CK20 (d) GCDFP-15 (A: ×400; B: ×200, C: ×200, D: ×100) |
Urethrectomy was performed and surgical pathology revealed a diffuse proliferation of cells with histological and immunohistochemical characteristics similar to the Paget cells observed in the glans biopsy. The cells were confined to the urothelium without invasion through the basement membrane (in situ urothelial carcinoma) and the surgical margins were free.
Paget′s disease was initially described by Crocker in 1889. [1] We were able to find only 15 previous reports of involvement of the glans associated with urothelial carcinoma. [2],[3],[4] The age ranged from 45 to 82 years (mean 61 years). In all but one case, the glans lesion was diagnosed after the urinary malignancy, after a mean interval of latency of 0.4 to 12 years (mean 6 years). In one patient, Paget′s disease of the glans allowed the diagnosis of an unsuspected transitional cell carcinoma of the bladder. [2] Most of the patients had aggressive tumors leading to death in 7 of the 15 patients.
Clinical presentation of Paget′s disease is often nonspecific (erosive, erythematous, eczematous, or circinate lesion) and can mimic many other dermatoses. Differential diagnoses include Bowen′s disease, Zoon′s balanitis, eczema, erythroplasia of Queyrat and psoriasis.
Microscopically, the two most important differential diagnoses include malignant melanoma and pagetoid Bowen′s disease. In melanoma we can see atypical melanocytes, which are positive for S100, HMB45, or Melan A. Pagetoid Bowen′s disease is a rare variant where the intraepithelial neoplastic squamous cells are arranged in nests, simulating the pattern of extramammary Paget′s disease. Epithelial mucins are negative in the neoplastic cells of pagetoid Bowen′s disease (in contrast, true Paget cells are positive for mucin stains). In general, the neoplastic cells of pagetoid Bowen′s disease are negative for CK7, CAM5.2, carcinoembryonic antigen (CEA), and epithelial membrane antigen (EMA).
Histopathological features in Paget′s disease of the breast and in secondary extramammary Paget′s disease are similar and in both these diseases, Paget′s cells are noted in a buckshot pattern. However, we have observed that Paget cells show a glandular or adenoid pattern in primary extramammary Paget′s disease., We believe this is an important histological clue to differentiate these disorders but immunohistochemistry is required for a more definitive diagnosis.
Immunohistochemical study can help to differentiate primary extramammary Paget′s disease from the secondary form, but this technique is not always diagnostic [Table - 1]. Kohler et al.[5] studied 6 cases of secondary extramammary Paget′s disease and 20 cases of the primary form. Positive GCDFP-15 in a patient with this entity may indicate low probability of associated internal malignancy. Ohnishi et al.[6] described 15 cases of primary and 13 cases of secondary extramammary Paget′s disease. They demonstrated that most secondary forms showed the immunophenotype CK7-/CK20+. Since they only analyzed seven cases of secondary disease, they suggested that the existence of CK7+/CK20- is a possibility in this entity. Additionally, CDX2 may be useful in discriminating primary extramammary Paget′s disease from secondary extramammary Paget′s disease following anorectal adenocarcinoma. CDX2 is positive when extramammary Paget′s disease is to colorectal adenocarcinoma but is usually negative when the disease is secondary to urothelial transitional cell carcinoma.
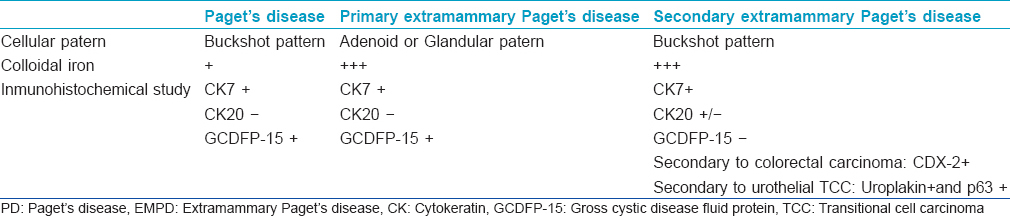
Finally, uroplakin is a transmembrane protein that is considered to be the most specific marker for urothelial differentiation. However, uroplakin III is reported to be expressed in 55% of cases. P63 is positive in 100% of conventional urothelial carcinoma. [7] Primary disease is uroplakin and p63 negative and the form secondary to urothelial transtitional cell carcinoma is uroplakin and p63 positive.
Despite the clinical similarities between primary and secondary extramammary Paget′s disease, treatment and prognosis are quite different. Prognosis of primary extramammary Paget′s disease is good but it has a high rate of recurrence after surgery due to its irregular and multicentric pattern. On the other hand, the prognosis and treatment of secondary extramammary Paget′s disease is determined by the nature of the internal malignancy and the presence or absence of metastatic disease. It is important to recognize secondary extramammary Paget′s disease because it may be the first manifestation of an occult carcinoma and because its co-existence indicates a worse prognosis than if the underlying carcinoma were present alone.
| 1. |
Jones RE Jr, Austin C, Ackerman AB. Extramammary Paget's disease: A critical reexamination. Am J Dermatopathol 1979;1:101-32.
[Google Scholar]
|
| 2. |
Salamanca J, Benito A, García-Peñalver C, Azorín D, Ballestín C, Rodríguez-Peralto JL. Paget's disease of the glans penis secondary to transitional cell carcinoma of the bladder: A report of two cases and review of the literature. J Cutan Pathol 2004;31:341-5.
[Google Scholar]
|
| 3. |
Somers K, Iorizzo L, Scott G, Mercurio MG. Extramammary Paget disease of the penis as a manifestation of recurrent transitional cell carcinoma. Dermatol Online J 2012;18:3.
[Google Scholar]
|
| 4. |
Kiyohara T, Ito K. Epidermotropic secondary extramammary Pagets disease of the glans penis from retrograde lymphatic dissemination by transitional cell carcinoma of the bladder. J Dermatol 2013;40:214-5.
[Google Scholar]
|
| 5. |
Kohler S, Smoller BR. Gross cystic disease fluid protein-15 reactivity in extramammary Paget's disease with and without associated internal malignancy. Am J Dermatopathol 1996;18:118-23.
[Google Scholar]
|
| 6. |
Ohnishi T, Watanabe S. The use of cytokeratins 7 and 20 in the diagnosis of primary and secondary extramammary Paget's disease. Br J Dermatol 2000;142:243-7.
[Google Scholar]
|
| 7. |
Paner GP, Annaiah C, Gulmann C, Rao P, Ro JY, Hansel DE, et al. Immunohistochemical evaluation of novel and traditional markers associated with urothelial differentiation in a spectrum of variants of urothelial carcinoma of the urinary bladder. Hum Pathol 2014;45:1473-82.
[Google Scholar]
|
Fulltext Views
3,873
PDF downloads
1,707





