Familial drug reaction with eosinophilia and systemic symptoms syndrome
Corresponding author: Dr. Asunción Vicente-Villa, Department of Dermatology, Hospital Sant Joan de Déu, Passeig Sant Joan de Déu 2, 08950, Esplugues de Llobregat, Barcelona, Spain. avicente@sjdhospitalbarcelona.org
-
Received: ,
Accepted: ,
How to cite this article: Morgado-Carrasco D, Fustà-Novell X, Mascaró JM Jr., Gonzalez-Enseñat MA, Vicente-Villa A. Familial drug reaction with eosinophilia and systemic symptoms syndrome. Indian J Dermatol Venereol Leprol 2021;87:383-5.
Sir,
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is an infrequent delayed hypersensitivity reaction to drugs characterized by rash, fever, eosinophilia, lymphadenopathy and systemic involvement. DRESS syndrome can even cause death and early suspension of the culprit drug is essential.1 There is a genetic predisposition.2 Here, we present a case of simultaneous DRESS syndrome in a father and son after starting antitubercular therapy.
A previously healthy 38-year-old South American man and his 9-year-old son was started on antitubercular treatment after they were both diagnosed with pulmonary tuberculosis. The father was given rifampin 600 mg/day, isoniazid 300 mg/ day, pyrazinamide 1600 mg/day and ethambutol 1100 mg/ day. The son weighed 31 kg and was started on rifampin 300 mg/day, isoniazid 150 mg/day, pyrazinamide 800 mg/day and ethambutol 550 mg/day. Two weeks later both presented with fever, odynophagia and pruritic skin lesions. On physical examination, they showed a generalized erythematous maculopapular rash [Figures 1a, b and c] and facial edema. Cervical, axillary and inguinal lymphadenopathies were found in both. Blood tests showed leukocytosis (29,600/ mm3 in father and 13,100/mm3 in son), eosinophilia (4,600/ mm3 in father and 2,800/mm3 in son) and elevation of liver transaminases (aspartate aminotransferase 552 IU/L in father and 67 IU/L in son, alanine aminotransferase 301 IU/L in father and 302 IU/L in son). Chest X-ray of the father revealed an interstitial pneumonitis. Blood cultures were negative. Serology (IgM and IgG) and polymerase chain reaction (PCR) on peripheral blood samples for herpes virus type 7, cytomegalovirus, Epstein Barr virus, measles, parvovirus B19 and mycoplasma pneumoniae were negative in both patients. The father showed a positive PCR for herpes virus type 6 but was negative in his son. He underwent a skin biopsy and histology showed an interface dermatitis and a perivascular inflammatory infiltrate with lymphocytes and eosinophils in the dermis [Figures 2a and b].
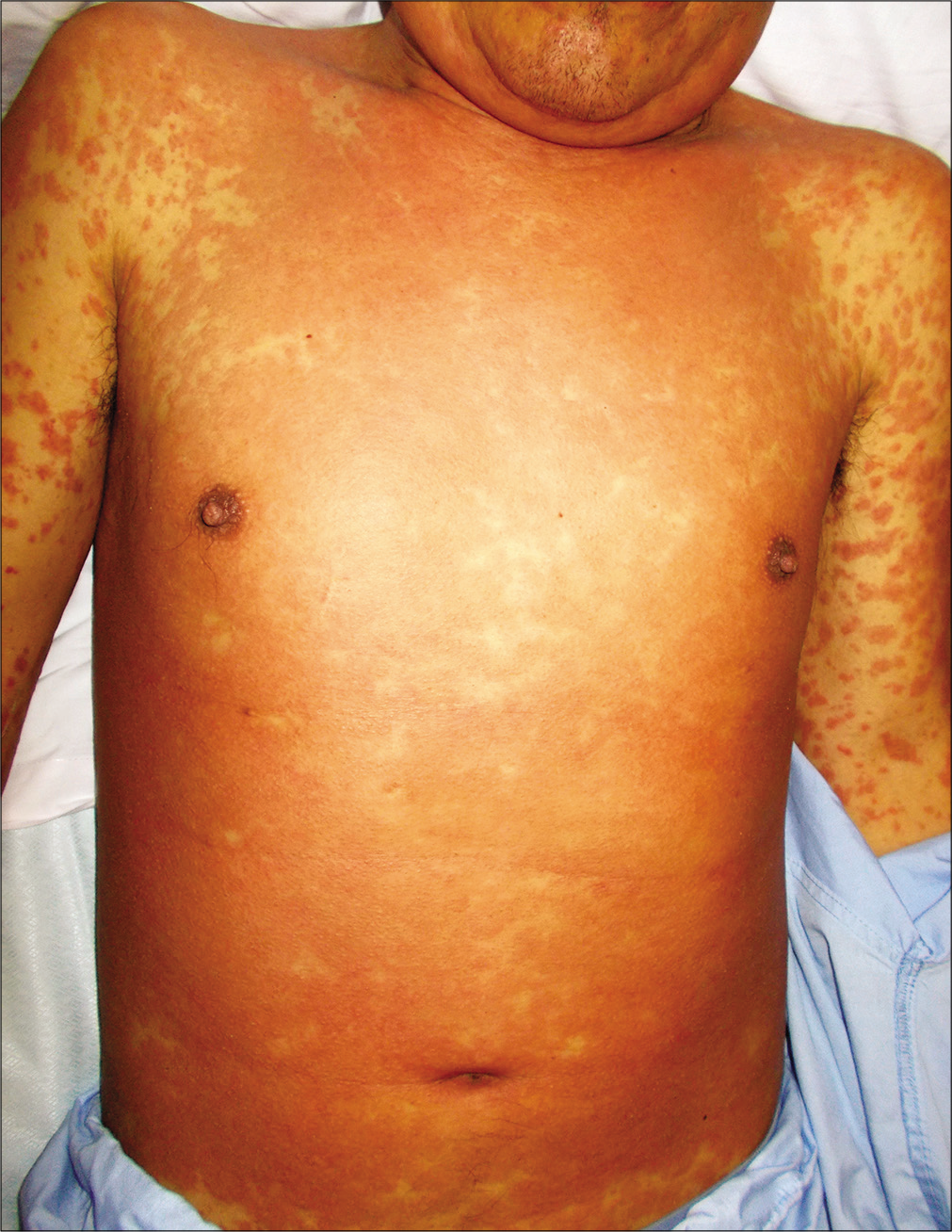
- Erythematous maculopapular rash on the father’s chest and upper limbs
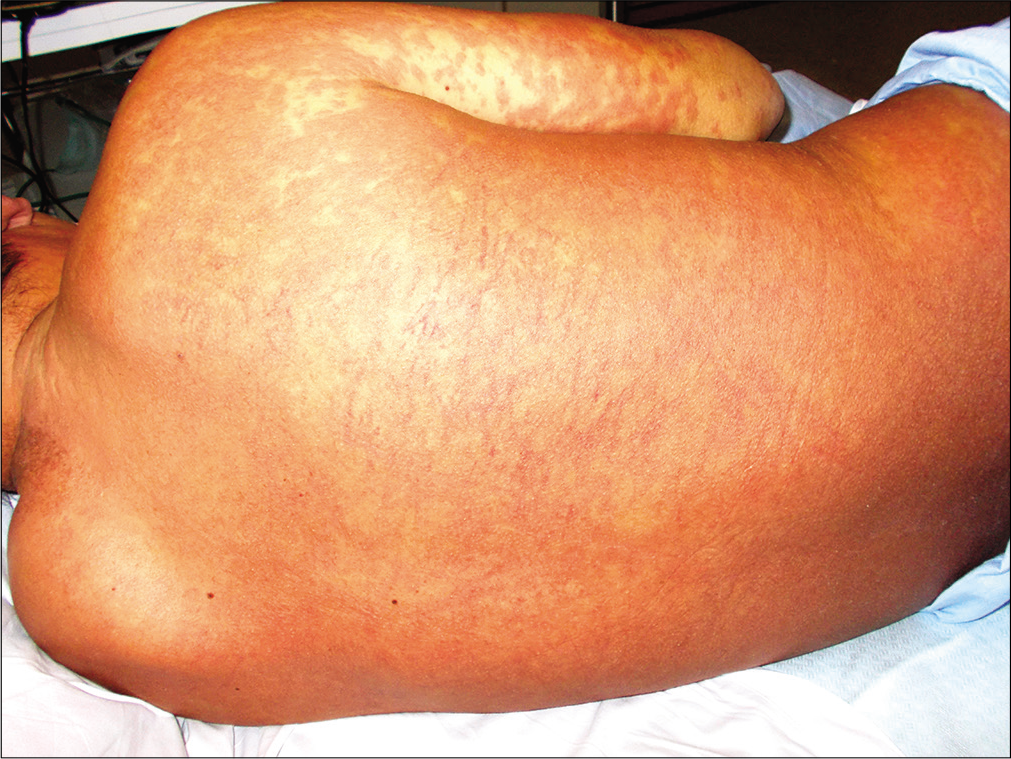
- Erythematous maculopapular rash on the father’s back
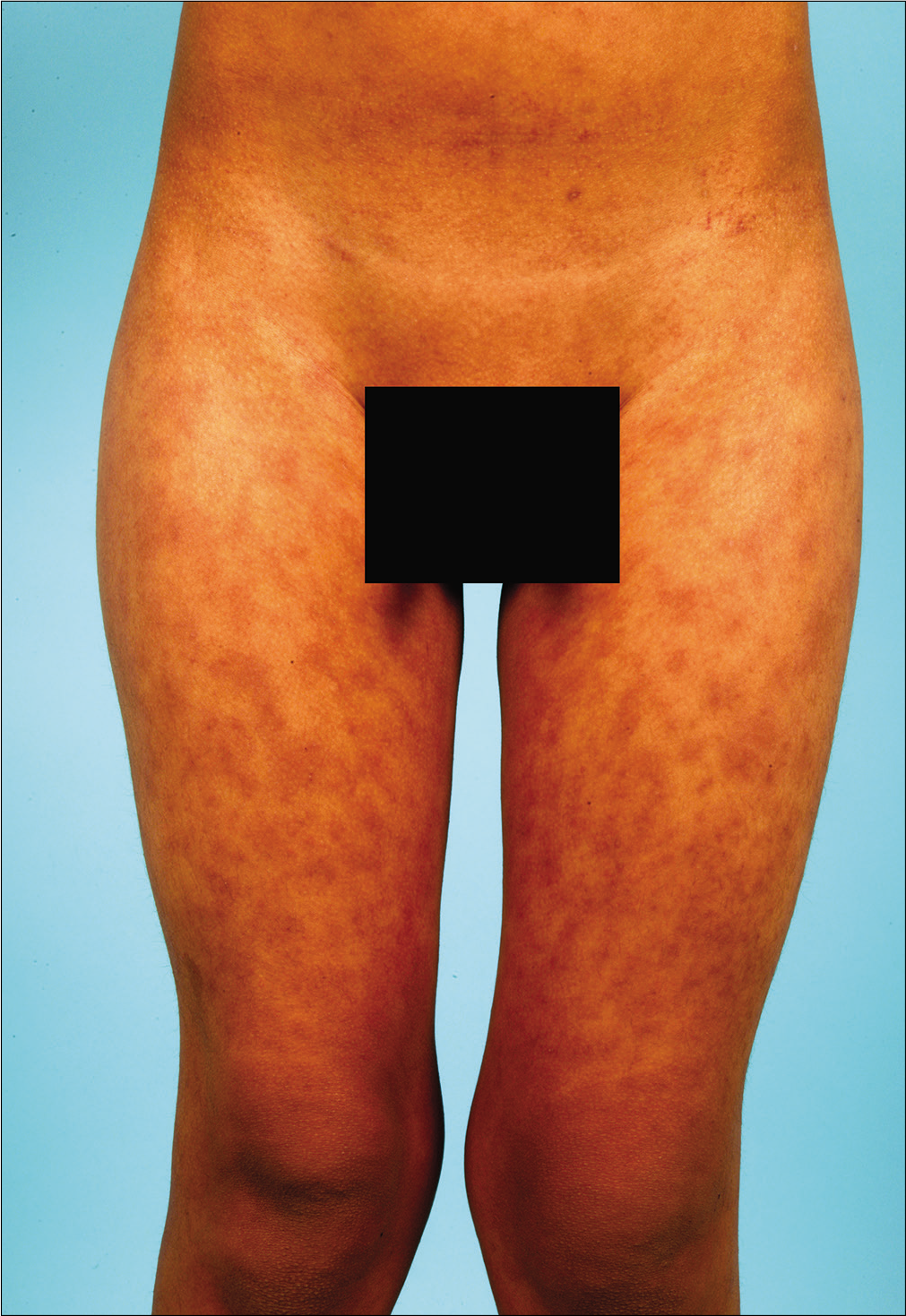
- Erythematous maculopapular rash on the son’s lower abdomen and anterior aspect of the thighs
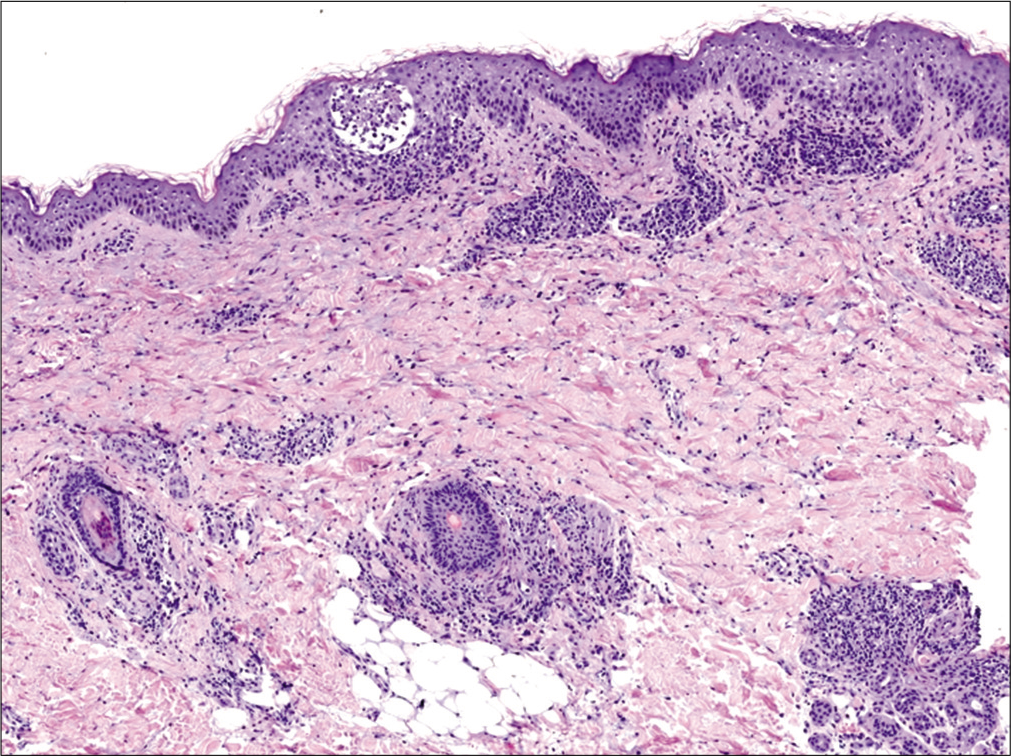
- Epidermal spongiosis, focal interface dermatitis and a perivascular and peri-appendageal inflammatory infiltrate with lymphocytes and eosinophils in the dermis (hematoxylin and eosin, ×40)
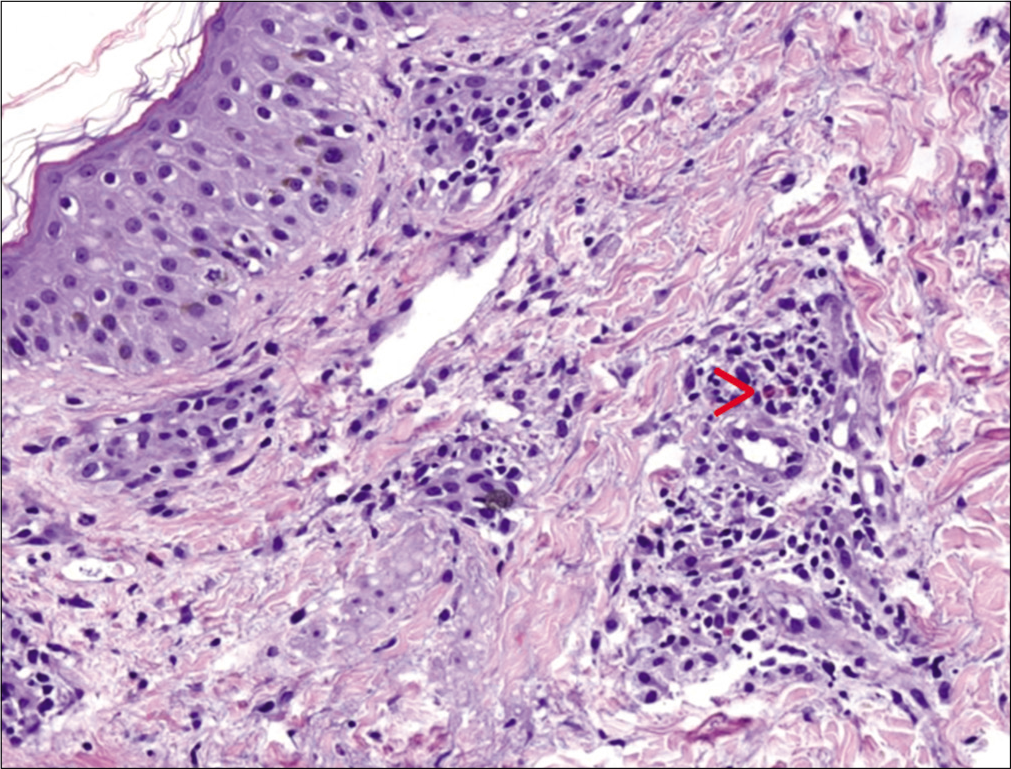
- Perivascular inflammatory infiltrate with lymphocytes and eosinophils in the dermis (red arrow) (hematoxylin and eosin, ×100)
Both patients fulfilled the criteria of the International Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) (8 and 6 points respectively) for definitive diagnosis (>5 points) of DRESS syndrome [Table 1].1
| Patient/age (years) | Culprit drug and dose (mg/day) | Time from drug intake to the onset of symptoms (days) | Clinical presentation | Blood tests and X-rays | Serology, PCR* and blood culture | PCR of **HHV-6 | Histological findings | RegiSCAR score*** |
|---|---|---|---|---|---|---|---|---|
| Father/38 | Rifampin: 600 Isoniazid: 300 Pyrazinamide: 1600 Ethambutol: 1100 |
15 | Fever Morbilliform rash affecting >50% BS**** (1 point) Facial and acral edema, purpura (1 point) Polyadenopathies (1 point) |
AST/ALT*****: 552/301 UI/L (liver involvement: 1 point) 4600×106eosinophils (2 points) Interstitial pneumonitis (lung involvement: 1 point) |
Negative (exclusion of other potential causes: 1 point) | Positive | Interface dermatitis, eosinophils in dermis | 8 (definitive diagnosis) |
| Son/9 | Rifampin: 300 Isoniazid: 150 Pyrazinamide: 800 Ethambutol: 550 |
15 | Fever Morbilliform rash Facial edema, purpura (1 point) Polyadenopathies (1 point) |
AST/ALT: 67/302 UI/L (liver involvement: 1 point) 2800×106eosinophils (2 points) |
Negative (exclusion of other potential causes: 1 point) | Negative | Not performed | 6 (definitive diagnosis) |
Antitubercular treatment was discontinued and oral corticosteroids were initiated (1 mg/kg/day). Both showed resolution of their symptoms and rash in the following 4 weeks and progressive normalization of laboratory tests during the following 8 weeks. The patients completed second-line antitubercular treatment with amikacin, levofloxacin and ethambutol without further complications. On genetic testing, the father did not present any known human leukocyte antigen (HLA) associated with DRESS syndrome (his HLA was DRB1*07, DRB1*08, A*02, A*24, B*40, B*44). The parents did not give consent to carry out genetic tests in the child. The patients were lost to follow-up and patch tests could not be performed.
The physiopathology of DRESS syndrome remains elusive. It involves reactivation of the herpesvirus family, altered immune response and genetic predisposition. Multiple HLA polymorphisms have been described, mostly in the Asian population, that confer greater susceptibility to severe hypersensitivity reactions. Interestingly, it seems that certain HLA variants are associated with specific drugs and specific cutaneous adverse reactions. HLA-A*31:01 and HLA-B*51:01 showed significant association with carbamazepine-induced DRESS,2 HLA-B*5801 has been linked to allopurinol-induced DRESS3 and HLA-B*5701 to abacavir-induced hypersensitivity reactions in both whites and blacks.4 The exact mechanism by which an HLA interacts with a drug and is capable of triggering an abnormal immune response is unknown. Only in the case of abacavir has a noncovalent binding between the drug and the HLA molecule been demonstrated. Such modifications of the immune presentation are endogenous and self-proteins induce polyclonal CD8 T-cell activation and a systemic reaction.5
Although adverse cutaneous effects of antitubercular drugs are frequent, DRESS syndrome is rarely seen. We could not find any report of HLA associated with antitubercular drug-induced DRESS but with our findings we suspect that an association could exist.
We have presented a rare case of a father and son who simultaneously developed a serious drug reaction, highlighting that there is a genetic predisposition for DRESS syndrome. Clinical suspicion was fundamental in excluding the differential diagnosis of a viral infection, a pathology that was more likely in contacts.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction, Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-80.
- [CrossRef] [PubMed] [Google Scholar]
- Genotype phenotype association between HLA and carbamazepine induced hypersensitivity reactions: Strength and clinical correlations. J Dermatol Sci. 2014;73:101-9.
- [CrossRef] [PubMed] [Google Scholar]
- HLA B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134-9.
- [CrossRef] [PubMed] [Google Scholar]
- High sensitivity of human leukocyte antigen b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis. 2008;46:1111-8.
- [CrossRef] [PubMed] [Google Scholar]
- Immune self reactivity triggered by drug modified HLA peptide repertoire. Nature. 2012;486:554-8.
- [CrossRef] [PubMed] [Google Scholar]





