Translate this page into:
Fever with rash in a child in India
2 Department of Pediatrics, Lady Hardinge Medical College and Kalawati Saran Children's Hospital, New Delhi, India
Correspondence Address:
Rashmi Sarkar
Department of Dermatology, Maulana Azad Medical College, New Delhi - 110 002
India
| How to cite this article: Sarkar R, Mishra K, Garg VK. Fever with rash in a child in India. Indian J Dermatol Venereol Leprol 2012;78:251-262 |
Abstract
Fever with rash is common among children and are seen by both dermatologists and pediatricians. Most of them are benign viral exanthems without much clinical significance. This article gives an overview of the infectious and noninfectious causes of fever with rash in children and how to diagnose them, with special emphasis on the Indian scenario as well. It also differentiates them from fever with rash caused by drugs. This review emphasizes that although benign in many cases, it is necessary to identify the signs of serious illnesses to prevent mortality or sequelae.Introduction
Fever with exanthem is common in childhood and a cause of anxiety among parents. Many of them are benign viral exanthems without much clinical significance. Reassurance and supportive therapy are all that is needed in such cases. However, a physician must be aware of the distinguishing features of the serious illnesses which may be associated with major complications with substantial morbidity and mortality.
There are a large number of infectious and noninfectious conditions presenting as exanthematous fevers. Many of them have a characteristic combination of clinical features. The rash is also morphologically different in different diseases. This review attempts to give an overview of the causes, epidemiology, salient clinical features, and diagnostic clues of fever with rash in the pediatric age group, particularly in the Indian context. The common causes of fever with rash in children are shown in [Table - 1].

Epidemiology
Viral exanthems are by far the most common cause of fever with rash in children. [1],[2],[3] There are a number of viruses causing various cutaneous manifestations associated with systemic symptoms. Rubeola or measles caused by a paramyxovirus is a major public health problem with significant mortality and morbidity, especially in developing countries. [4] The incidence of the disease has declined substantially in industrialized countries. [5] Measles is transmitted by droplet infection and commonly affects children between 6 months and 3 years of age, with a trend toward higher age group in the developed countries. [6] In India, epidemics occur in winter and early spring. The number of cases have decreased (i.e., 40,840 cases and 44 deaths in 2009 [7] ) in the country since the implementation of Universal Immunization Programme.
Another life-threatening exanthematous fever in children which deserves special mention is dengue fever. The arbovirus transmitted by Aedes egypti mosquito causes explosive epidemics in the monsoon season in India. There has been an increasing trend over the years with 15,509 cases, having a case fatality rate of 0.57 in 2009. The disease occurs in all ages and both sexes are equally affected. [8]
Another dengue-like illness is chikungunya fever, which is also transmitted by the same vector and in the same season. Its incidence is also on the rise in India with a massive epidemic occurring in 2006 affecting nearly 1.39 million cases spread over 213 districts in 15 states. [9],[10],[11]
On the other hand, most of the other viral exanthems are self-limiting without serious complications. Herpes simplex infections are ubiquitous without any seasonal variation. Infected individuals harbor latent infection responsible for recurrences, with highest seroprevalence seen in the developing countries. [12] Varicella and rubella mainly affect children between 3 and 10 years of age, both being transmitted by droplet infection. [13],[14] human herpes virus 6 (HHV6) and Epstein-Barr virus (EBV) infections occur in infancy and early childhood. [3],[15] By the end of the second year, almost 100% children are seropositive for HHV6. [3] Both viruses are transmitted by contaminated saliva. Enteroviruses, known to cause numerous exanthems in children, occur round the year in tropical and subtropical areas. They are highly contagious, transmitted via contact with nasal/oral secretions, aerosol droplets and fecal material. Infants less than 1 year of age account for more than 25% of the cases with high incidences seen among male sex and conditions with poor hygiene and low socioeconomic status. Epidemics of hand foot and mouth disease (HFM) are caused by coxsackievirus A16 or Enterovirus71, whereas coxsackievirus A9, A10, B1, through B3 and B5 cause sporadic cases. [14]
Parvovirus B19 infections occur mainly in winter and spring transmitted via respiratory droplets. The classic exanthem of this virus is erythema infectiosum (EI) which is most common between 4 and 10 years of age. The other exanthem caused by the same virus is Papular purpuric gloves and socks syndrome (PPGS), which occurs mainly in young adults. [2]
Cutaneous eruptions with fever also occur in many bacterial infections. Children are known to have various staphylococcal skin infections without much clinical significance. However, staphylococcal scalded skin syndrome (SSSS) and toxic shock syndrome (TSS) are potentially fatal conditions caused by the same organism. SSSS or Ritter′s disease occurs predominantly in infants and children less than 5 years of age. TSS occurs mostly in menstruating girls between 15 and 25 years, but is also seen in nonmenstruating girls and children where the infection occurs from nasal packs, wounds, sinusitis, tracheitis, etc. [16]
Group A streptococci (GAS) is responsible for causing scarlet fever (SF), erysipelas, and acute rheumatic fever (RF), each of which is associated with high fever and characteristic rash. [17] The incidence of SF is maximum between 2 and 10 years. [18],[19] RF affecting children between 5 and 15 years of age has been reported to occur in 1-3% of those with prior streptococcal infections. [20]
The incidence of endemic meningococcal disease is low in India with occasional outbreaks, mainly in the cities of north India, usually occurring in winter and almost always caused by the serogroup A. [21],[22] Highest incidence is seen in children younger than 5 years with a second peak between 15 and 24 years. Most infections are acquired from a colonized adult or a patient with meningococcal disease. [23],[24],[25]
Leptospirosis has been known to occur in India since long, especially in areas with heavy monsoon, animal rearing practices, and unplanned urbanization. [26],[27],[28],[29],[30] Outbreaks have been reported from Orissa after the supercyclone in 1999 and from Mumbai in 2000 and 2005. [28] As many as 232 cases have been reported from a hospital in north India between 2004 and 2008. [26] Children usually acquire this infection by direct or indirect contact with urine of infected dogs. [28]
A discussion on infectious causes of fever with rash is incomplete without mentioning the myriad of rickettsial diseases [Table - 1], which are largely underdiagnosed, but are an important cause of febrile rash in children in India. [31],[32],[33] Transmission can be via mites, rat fleas, louse, or tick.
Noninfectious causes of febrile rash include drug eruptions and collagen vascular diseases (CVD). Adverse cutaneous drug eruptions occur in approximately 2-3% of hospitalized patients. [34],[35] Most of them are mild and self-limited, resolving with the withdrawal of the offending drug. Severe and potentially life-threatening eruptions occur in approximately 1 in 1000 hospitalized patients. [36] Of these, toxic epidermal necrolysis (TEN) has been reported to have high mortality rates ranging from 20 to 70%. [37]
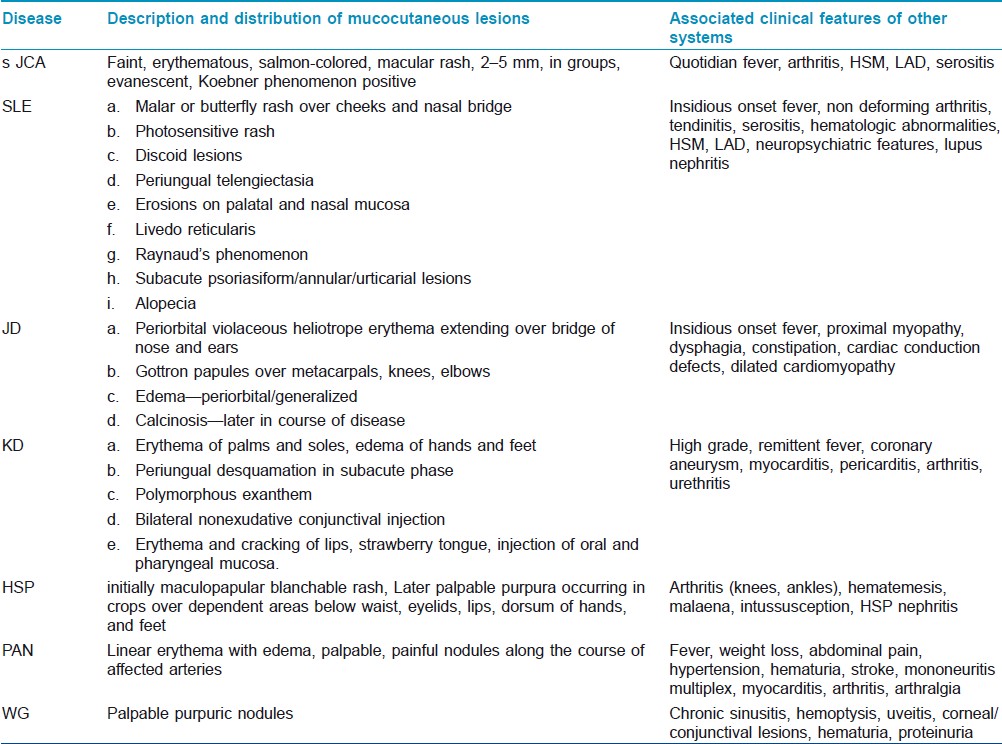
Notable among the CVD are systemic lupus erythematosus (SLE), systemic onset juvenile chronic arthritis (sJCA), and vasculitis. Lupus has a female preponderance, female to male ratio being 4:1 before puberty and 8:1 after puberty. Onset of the disease is usually after 8 years and its prevalence varies from 4 to 250/1,00,000. [38] sJCA is commonly seen in young children often less than 1 year of age with no sex predilection. [39] Among the vasculitis, Kawasaki disease (KD) and Henoch-Schonlein purpura (HSP) are worthy of mention. KD has increasingly been reported from different parts of the world since its first publication in English medical literature in 1974. [40] Prevalence of KD is highest among Asians, intermediate in blacks, and lowest in whites. [41],[42],[43] 85% of the cases are below 5 years of age. It is underreported in India; [44],[45] the true incidence is expected to be higher and there are increasing reports from centers in India that KD has replaced HSP as the commonest vasculitic disorder in hospitalized children. [46],[47] HSP is a common cause of hospital admission in children with an overall estimated incidence of 9/100,000, with a peak age of 2-8 years and male to female ratio of 2:1. [48],[49] The other CVD like juvenile dermatomyositis (JD), Wegener′s granulomatosis (WG), and polyarteritis nodosa (PAN) are relatively less common. The occurrence of JD and WG in girls is two to three times more common than boys with a mean age of 6-7 years. [50],[51] PAN occurs equally in boys and girls with a mean age of 9 years. [52]
Clinical Features
Many of the above mentioned causes have a characteristic combination of signs and symptoms. Measles is characterized by a distinct prodromal phase with fever, rhinorrhea, cough, and conjunctival congestion. Koplik spots consisting of gray white sand-like lesions with surrounding erythema in the buccal mucosa opposite the lower second molar tooth are pathognomonic. Erythematous, confluent, maculopapular rash develops usually on the fourth day of fever, beginning behind the ears and forehead and progressing downward. The rash resolves in the same order with residual brownish discoloration and desquamation which fades over the next 10 days. [53]
The rash of rubella consists of minute, discrete macules, in contrast to the confluent rash of measles. The rash appears within 24 hours of onset prodromal symptoms, spreads rapidly from face to trunk and extremities and disappears altogether by the third day. Posterior cervical and postauricular lymphadenopathy, although not pathognomonic, is commonly seen. [3],[14]
On the other hand, the rash of varicella occurs in crops, which evolves through the stages of macule, papule, vesicle, and crusts involving mainly the trunk and proximal end of extremities [Figure - 1]. Fever subsides 2-4 days after appearance of rash and the rash disappears after 3-7 days, leaving behind hypo- or hyperpigmented macules persisting for days to weeks. [13],[54]
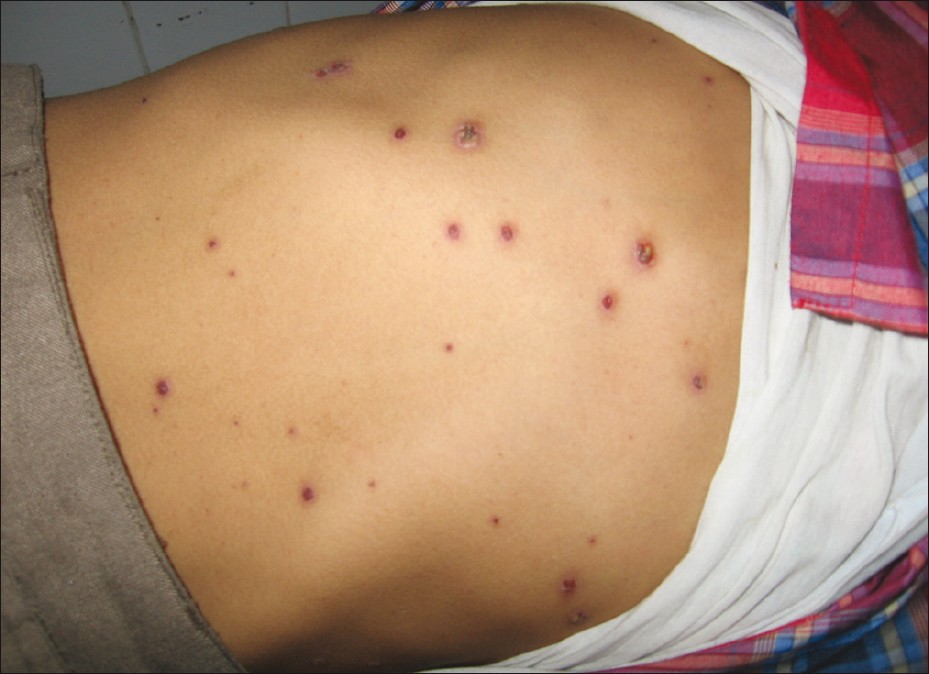 |
| Figure 1: Pleomorphic eruption of varicella with vesicular and crusted lesions over trunk |
In infectious mononucleosis, fever, pharyngeal inflammation, generalized lymphadenopathy, and hepatosplenomegaly are associated with a maculopapular rash which may take the form of erythema multiforme or urticaria. The exanthem occurs in 3-13% of cases and increases to 50% if treated with ampicillin or amoxicillin. [15],[55],[56]
Children with dengue fever present with sudden onset of high grade fever associated with facial flushing, headache, myalgia, arthralgia, and abdominal pain. Within 48 hours of onset of fever, a transient generalized macular rash develops. Fever may subside after 2-7 days, only to reappear after 1-2 days, demonstrating the typical biphasic pattern. The critical phase begins at defervescence, marked by some warning signs like intense abdominal pain, persistent vomiting, mucosal bleeding, clinical fluid accumulation, lethargy, and tender hepatomegaly. This second phase is associated with a generalized morbilliform maculopapular rash sparing the palms and soles [Figure - 2]. The extent of plasma leakage decides whether patient recovers from this phase or progresses to the next phase of severe dengue. Severe dengue is defined by one or more of the following: (a) shock from plasma leakage; (b) severe bleeding; and (c) severe organ impairment. [57],[58]
 |
| Figure 2: Morbilliform maculopapular rash of dengue |
Chikungunya fever is characterized by high fever with rigors, accompanied by intense joint pain, headache, myalgia, and rash. The arthritis is polyarticular, migratory involving small joints. The joint pain tends to persist for long. The rash is itchy, transient, maculopapular, distributed over trunk, and extremities, appearing 4-8 days after fever and arthritis. [9],[10],[11],[59]
The lesions of herpes simplex types 1 and 2 are in the form of single or grouped vesicles varying from 2 to 10 mm, on a mildly erythematous base. Fever blisters are the most common presentation of recurrent HSV-1 infection, primarily seen over lips, nose, chin, cheek, or oral mucosa. Occasionally, erythema multiforme or Steven-Johnson Syndrome may be seen. [12],[53]
Children infected with HHV6 exhibit a sudden high fever lasting for 3-5 days. With cessation of fever, discrete macular or maculopapular rash erupt, known as exanthem subitum (ES), over neck and trunk which begins to disappear in a few hours. These children have a 10% risk of febrile convulsions. Pityriasis rosea (PR), commonly affecting adolescents and young adults, has been considered to be associated with HHV6 and HHV7. After a prodromal period, the rash begins with a primary or "herald patch." Within 1-2 weeks, morphologically identical but smaller lesions appear on the trunk and proximal extremities. The lesions are distributed along the lines of cleavage and have a ring of scales surrounding them. [2],[3]
EI caused by Parvovirus B19 is seen as a homogenous erythema over the cheeks demonstrating the typical "slapped cheek" appearance, associated with a reticulate maculourticarial exanthem over the proximal extremities and occasionally over distal extremities and trunk. The rash undergoes repeated fading and recurrence triggered by local irritants, high temperature, and emotional stress. Parvovirus B19 also causes PPGS which consists of edema and erythema of both hands and feet with associated petechiae and purpura. The eruption may extend on to dorsal surfaces but ends abruptly at wrists and ankles and is frequently associated with burning and itching. Oral erosions, vesicles, lip edema, and petechiae over hard palate, pharynx, and tongue may also be seen. [1],[3],[14],[54]
Enteroviruses are responsible for a large number of exanthems. HFM disease is most common. After a prodrome of 2-4 days, large (2-8 mm), oval, gray blisters with erythematous base develop over hands, feet, and buttocks. Painful aphthous ulcers are seen in the mouth. The rash resolves in 5-7 days. [14],[54]
Gianotti-Crosti syndrome, also called papular acrodermatitis of childhood, affects children between 1 and 6 years of age. Many viruses have been implicated to cause this disease, namely hepatitis B, EBV, cytomegalovirus, and coxsackievirus. The rash that follows a prodrome of fever and respiratory symptoms is composed of symmetric, homogenous, flat-topped pink-brown papules distributed classically on cheeks, extensor extremities, and buttocks. Hemorrhagic or moist eczematous papules may also be seen. The rash lasts for around 15-20 days, sometimes longer. [1],[2],[14]
Staphylococcal infections in children range from furuncles, carbuncles, folliculitis, bullous impetigo (which may or may not be associated with fever) to TSS and SSSS (which are associated with high temperatures). TSS is an acute multisystem disease characterized by high fever, vomiting, diarrhea, conjunctival congestion, strawberry tongue and diffuse, dark, and sunburn-like rash. This is accompanied by altered sensorium, disseminated intravascular coagulation, and hypotension. Symptoms resolve by 7-10 days, associated with desquamation of palms and soles. SSSS affecting predominantly children below 5 years consists of a scarlatiniform erythema transforming into a wrinkled paper appearance with peeling of large sheets of epidermis. Healing occurs within 10-15 days without scarring. [16],[60],[61]
SF caused by GAS is characterized by a rash and strawberry tongue appearing 1-2 days after an upper respiratory tract infection. The rash is typically diffuse, erythematous, blanchable, and finely papular (like sandpaper) beginning around the neck spreading over trunk and extremities, sparing the face. The rash fades with desquamation after 3-4 days. [18],[19] Erysipelas, also caused by GAS, is a well-circumscribed, indurated, tender rash arising abruptly with high fever. Erythema marginatum is one of the major diagnostic criteria of acute RF, which is a nonsuppurative complication of prior GAS infection. The rash consists of erythematous, serpiginous, macular, nonpruritic rash with central pallor, primarily occurring over trunk and extremities, sparing the face. [17]
Enterococcal and viridans group of streptococci are known causes of bacterial endocarditis, cutaneous manifestations of which include petechiae, splinter hemorrhages, Osler′s nodes, and Janeway′s spots. [53]
Meningococcemia is characterized mainly by fever with petechiae, but other transient lesions resembling a viral maculopapular rash may also be seen. The petechiae are often raised with a "smudged" appearance involving trunk and extremities. Gangrenous hemorrhagic areas resembling purpura fulminans are seen in fulminant meningococcemia which is also associated with adrenal hemorrhage, hypotension, multiorgan failure (MOF) with or without meningitis. [21],[22],[23],[25],[62]
Disease with Yersinia enterocolitica manifests as diarrhea and enterocolitis and infrequently septicemia. Erythema nodosum, arthritis, and uveitis may develop as reactive complications. Septicemic plague caused by Yersinia pestis presents with initial generalized erythema followed by petechiae and purpura. [63]
The clinical features of leptospirosis encompass a wide spectrum ranging from asymptomatic infection at one extreme to MOF at the other. Symptomatic children present with a biphasic pattern, beginning with abrupt onset of high fever with chills, headache, severe tenderness over lower half of the body, conjunctival suffusion, hepatosplenomegaly (HSM), and generalized lymphadenopathy (LAD). After a brief period of well-being, the second phase occurs characterized by a transient (<24 hours) rash in 10% of cases. The rash may be urticarial, petechial-purpurial or desquamating type. Meningitis and uveitis may occur in this phase. [64],[65]
Borrelia infections are known to cause fever with rash. Notable among them are Lyme disease and relapsing fever. Erythema migrans, an annular rash, is typical of Lyme disease. It may be uniformly erythematous or may take the form of a target lesion with necrosis and vesicles in the center. Axilla, periumbilical area, thigh, and groin are the usual sites. The rash enlarges to average 15 cm with a sharply demarcated peripheral reddish band about 1-2 cm wide and resolves without treatment in 4 weeks. Relapsing fever consists of recurrent febrile and afebrile periods, each lasting 2-7 days. Usually the terminal part of the primary febrile episode is associated with a diffuse, erythematous macular, or petechial rash over trunk and shoulders lasting 1-2 days. Jaundice, splenomegaly, lymphadenopathy, and CNS features may also be seen. [66]
Trench fever, caused by Bartonella quintana is characterized by fever for about a week, followed by recurrent fevers, usually every 5 days, associated with a nonspecific macular rash, severe headache, neck, back, and shin pain. [67]
Rickettsial fevers usually have varying combination of features including fever, headache, myalgia, hepatosplenomegaly, and lymphadenopathy along with rash. The rash is macular or maculopapular, occasionally petechial. The diseases may be associated with a typical painless eschar with an erythematous rim at the site of vector bite. [31],[32],[33],[68]
Drug eruptions, although mostly morbilliform and exanthematous, can present with various patterns, the knowledge of which can help the clinician determine the causative medication and the most appropriate treatment. Acute generalized exanthematous pustulosis (AGEP) is characterized by acute-onset fever associated with generalized scarlatiniform erythema and many small, sterile, nonfollicular pustules. Antibiotics are the predominant cause, others being mercury exposure or UV radiation. AGEP resolves spontaneously by 7-10 days followed by desquamation over a few days. [69]
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome or drug-induced hypersensitivity syndrome, usually caused by anticonvulsants, is characterized by the triad of fever, skin eruption, and internal organ involvement. [70]
Stevens-Johnson syndrome (SJS) is a serious mucocutaneous illness with systemic symptoms characterized by the presence of flat, atypical target lesions [Figure - 3], epidermal detachment comprising less than 10% of the total body surface area (BSA) and involvement of two or more mucosal sites. TEN is a life-threatening condition characterized by high fever associated with widespread (involving more than 30% of BSA) confluent erythema followed by necrolysis. An epidermal involvement between 10-30% of BSA has been referred to as SJS-TEN overlap. Anticonvulsants, especially carbamazepine and phenytoin, are most frequently implicated in causing SJS and TEN. Other drugs include antibiotics like sulphonamides, penicillins, and fluoroquinolones as well as
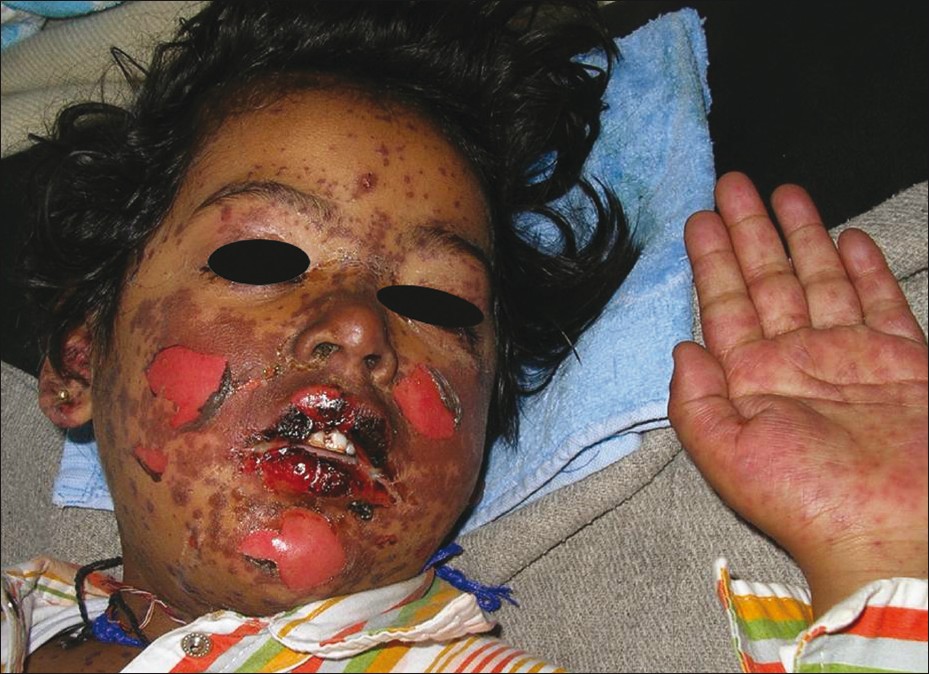 |
| Figure 3: Steven Johnson Syndrome showing dusky macules, papules with oral mucosal involvement and hemorrhaging crusting over the lips, following intake of phenytoin |
Another severe drug eruption includes leucocytoclastic vasculitis, characterized by blanching erythematous macules, replaced later by palpable purpura. Fever, myalgias, arthritis, and abdominal pain may be present. It typically appears 7-21 days after the onset of drug therapy. [73]
Antibiotics have also reported to cause serum sickness and serum sickness-like cutaneous eruptions which begin with erythema on the sides of the fingers, hands, and toes and progress to a widespread morbilliform or urticarial eruption. Multiorgan involvement, fever, arthralgia, and arthritis are common. [74]
In contrast to drug-induced and infectious febrile rashes, CVDs are usually characterized by insidious onset, low-grade, indolent fever with nonspecific constitutional symptoms like fatigue, anorexia, and weight loss. However, high spiky fever is seen in sJCA and KD. Children with KD present with a remittent or continuous fever for about 1-2 weeks without a prodrome of respiratory symptoms. The acute phase is characterized by erythema and/or edema of hands and feet, polymorphous erythema over body and extremities, bilateral nonexudative conjunctival injection, redness, dryness and fissuring of lips, a strawberry tongue, and nonsuppurative cervical lymphadenopathy. Fever and all the acute signs subside in the subacute phase, except conjunctivitis. This phase (lasting for about 2 weeks) is associated with desquamation, thrombocytosis, and coronary aneurysms [Figure - 4]. Convalescent phase begins with disappearance of all clinical features and lasts for the next 2-4 weeks till ESR and CRP normalize. [46],[47] The clinical manifestations of the different CVDs have been summarized in [Table - 2].
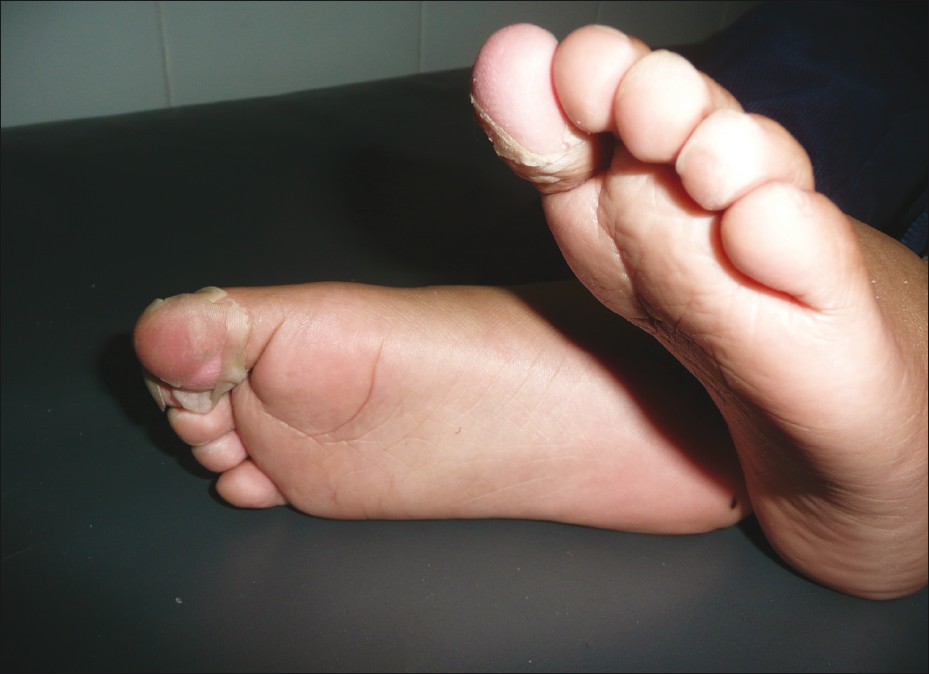 |
| Figure 4: Subacute phase of Kawasaki disease showing desquamation over toes |
Diagnostic Approach
A febrile child with rash often presents a diagnostic challenge for physicians. The distinct morphological features and distribution of the rash along with a characteristic cluster of systemic features may give a clue to the clinician. A thorough history including onset, duration and type of fever, temporal association between fever and rash, sequence of distribution of rash, associated symptoms, presence of similar lesions in close contacts, recent intake of medicines, and hygiene status of the household should be taken. Careful physical examination entails close examination of the rash and salient features of systemic involvement.
Age
Age of the child can give some idea regarding probable etiologies. Viral exanthems like measles, ES, EBV, and enteroviral infections commonly occur in infants and children below 3 years of age. Most of the cases of KD and SSSS are children below 5 years of age. The age of occurrence of varicella, rubella, and EI overlaps with that of the above mentioned conditions, extending from 3 to 10 years of age. Meningococcemia shows two peak ages of affection-children less than 5 years and between 15 and 24 years. SLE, JD, RF, PAN, and WG affect older children above 6-7 years while HSP occurs between 2 and 8 years and sJCA usually seen in less than 1 year. On the other hand, dengue and chikungunya fevers affect all age groups.
Morphology of Rash
The differential diagnoses based on morphology of rash is shown in [Table - 3]

Associated Clinical Features
It cannot be more emphasized that the type of rash and clinical features associated with the rash point to the diagnosis making confirmatory laboratory diagnosis unnecessary in many instances. An etiological approach based on associated systemic features has been shown in [Table - 4].
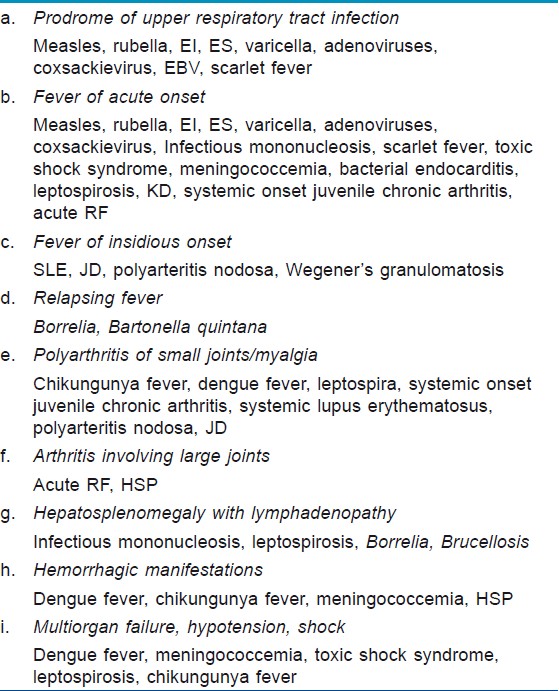
Prodrome of upper respiratory tract infection
A prodrome of malaise, fever, catarrhal symptoms, and sore throat preceding the rash usually suggests viral exanthems like measles, rubella, ES, EI, varicella, coxsackievirus, EBV, and adenoviral infection. The distinguishing features of these infections have been discussed earlier. Virological confirmation is not necessary.
Diseases mimicking viral exanthems
KD is often misdiagnosed as viral infections due to common features. This can be a serious issue as untreated KD may develop coronary aneurysms which may remain undiagnosed. KD is diagnosed by a set of clinical parameters without any specific laboratory investigation criteria. [75] Absence of prodrome of respiratory symptoms, nonexudative conjunctivitis, distribution of the rash predominantly over the trunk, edema over hands and feet with desquamation, and a raised ESR, differentiate KD from the above mentioned viral infections. A high index of suspicion and experience is required to identify atypical cases of KD which have fever with few of the characteristic clinical features, but develop coronary aneurysm. [76],[77] SF is sometimes difficult to differentiate from KD, both having scarlatiniform rash and strawberry tongue. Absence of rapid clinical response to antistreptococcal antibiotics within 24-48 hours would go in favor of KD. The typical transient nature of rash with Koebner phenomenon differentiates sJCA from viral exanthems, KD, leptospirosis, and SF.
Drug-induced eruptions are very common and often difficult to differentiate from viral exanthems. The former are more likely to be urticarial, intensely erythematous and pruritic, and may or may not be associated with the prodromal features of viral fevers. SJS involving skin and mucous membrane mimics SF or KD. In SJS, erythematous macules develop central necrosis resulting in vesicles, bullae, and denuded areas. Such vesiculobullous lesions are absent in SF or KD. TEN, also triggered by drugs, consists of sudden onset (within 24-48 hours) widespread blister formation, confluent or morbilliform erythema, skin tenderness, and positive Nikolsky sign. A temporal relationship between appearance or worsening of rash and intake of drugs, morphology of the rash, its characteristic pruritic nature, and presence of eosinophilia in blood are suggestive of drug-induced rash. Sometimes biopsy can be helpful in confirming the diagnosis of a drug eruption (e.g., by showing eosinophils in morbilliform eruptions). [73]
Circulatory failure
Dengue and chikungunya are two of the conditions [Table - 4] which may be associated with shock. Both are noted for absence of respiratory symptoms. In fact, Ramos et al. concluded that presence of high fever, petechial rash, or mucosal bleed in the absence of cough and other respiratory symptoms has a very high positive predictive value of confirmed dengue infection. [78] Headache, myalgia, and arthralgia are seen in both diseases, with polyarticular arthralgia involving small joints and pruritic maculopapular rash more suggestive of chikungunya fever. Progressive leukopenia and a positive torniquette test are the earliest markers of probable dengue. Meticulous monitoring for warning signs described earlier can identify progression to critical phase before clinical signs of plasma leakage appear. Severe dengue consisting of shock, hemorrhage, and MOF must be differentiated from conditions with similar features like malaria, leptospirosis, and meningococcal or other bacterial shock. The hemorrhage in fulminant meningococcemia usually is in the form of purpura fulminans unlike dengue where gastrointestinal bleeding, petechiae, and purpura predominate. Although tender hepatomegaly may be seen in dengue, associated splenomegaly is suggestive of malaria and additional lymphadenopathy would indicate leptospirosis. Confirmation of diagnosis can be done by blood counts, blood culture for meningococcemia, peripheral smear and rapid antigen tests for malaria, serological tests for dengue, chikungunya, and leptospira.
Another exanthematous fever-associated circulatory failure includes TSS caused by Staphylococcus aureus and occasionally GAS. Females between 15 and 25 years of age are common victims with the typical sunburn-like rash and MOF as described earlier. Diagnosis is primarily clinical.
Insidious onset fever
Insidious onset fever, relatively higher age of presentation, and characteristic distribution of rash [Table - 2] allow easy identification of CVD like SLE, JD, PAN, and WG. Diagnostic criteria of SLE are well established. [79] Typical clinical features, a positive antinuclear antibody (seen in more than 80% of cases), anti-Pm/Scl, and characteristic muscle biopsy findings confirm the diagnosis of JD. Typical findings of vasculitis on biopsy or angiography confirm PAN while antibodies to ANCA directed toward PR3 are specific for WG.
Conclusion
Exanthematous fevers in children have an exhaustive list of causes. A stepwise approach consisting of proper history taking, identifying the morphological type of lesion, and ultimately correlating the two in the background of appropriate clinical mileu will go a long way in identifying the etiology and ordering the appropriate investigations.
Acknowledgement
We wish to acknowledge Dr. Srikanta Basu, Associate Professor, Dept of Pediatrics, Lady Hardinge Medical College and KSCH, New Delhi for [Figure - 2] and [Figure - 3].
| 1. |
Manchani AJ. Exanthems in Childhood: An Update. Pediatr Ann 1998;27:163-70
[Google Scholar]
|
| 2. |
Nelson JSB, Stone MS. Update on selected viral exanthems. Curr Opin Pediatr 2000;12:359-64.
[Google Scholar]
|
| 3. |
Fölster-Holst R, Kreth HW. Viral exanthems in childhood - infectious (direct) exanthems. Part 1: Classic exanthems. J Dtsch Dermatol Ges 2009;7:309-16.
[Google Scholar]
|
| 4. |
WHO-UNICEF joint statement on strategies to reduce measles mortality worldwide. Geneva: World Health Organization. [homepage on the Internet] 2001. Available from: http://www.who.int/vaccines-documents/DocsPDF01/www667.pdf.
[Google Scholar]
|
| 5. |
Gindler J, Tinker S, Markowitz L, Atkinson W, Dales L, Papania MJ. Acute measles mortality in the United States, 1987-2002. J Infect Dis 2004;189:69-77.
[Google Scholar]
|
| 6. |
Cottrell S, Roberts RJ. Measles outbreak in Europe. BMJ 2011;342:3724.
[Google Scholar]
|
| 7. |
Central Bureau of Health Intelligence, National Health Profile (NHP) of India - 2009. [homepage on the Internet] 2009. Available from: Government of india, Director general of health services. ministry of health and family welfare Web site: http://cbhidghs.nic.in/index2.asp?slid=1068&sublinkid=925. [Last cited 2011 Sep 5].
[Google Scholar]
|
| 8. |
Central Bureau of Health Intelligence, National Health Profile (NHP) of India - 2010. [homepage on the Internet] 2010. Available from: Government of india, Director general of health services. ministry of health and family welfare Web site: http://cbhidghs.nic.in/index2.asp?slid=1068&sublinkid=925. [cited 2011 Sep 5].
[Google Scholar]
|
| 9. |
Krishnamoorthy K, Harichandrakumar KT, Krishna Kumari A, Das LK. Burden of chikungunya in India: Estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J Vector Borne Dis 2009;46:26-35.
[Google Scholar]
|
| 10. |
Mavalankar D, Shastri P, Bandyopadhyay T, Parmar J, Ramani KV. Increased mortality rate associated with chikungunya epidemic, Ahmedabad, India. Emerg Infect Dis 2008;14:412-5.
[Google Scholar]
|
| 11. |
Seyler T, Hutin Y, Ramanchandran V, Ramakrishnan R, Manickam P, Murhekar M. Estimating the burden of disease and the economic cost attributable to chikungunya, Andhra Pradesh, India, 2005-2006. Trans R Soc Trop Med Hyg 2010;104:133-8.
[Google Scholar]
|
| 12. |
Salvaggio MR, Lutwick LI, Seenivasan M. Herpes simplex. [homepage on the Internet] 2010. Available from: http://emedicine.medscape.com/article/218580. [cited 2011 Nov 2].
[Google Scholar]
|
| 13. |
Arvin AM. Varicella-zoster virus. Clin Microbiol Rev 1996;9:361-81.
[Google Scholar]
|
| 14. |
Dyer JA. Childhood viral exanthems. Pediatr Ann 2007;36:21-9.
[Google Scholar]
|
| 15. |
Wong D. Herpes simplex virus. [homepage on the Internet]. Available from: http://virology-online.com/viruses/HSV.htm. [cited 2011 Nov 3].
[Google Scholar]
|
| 16. |
Manders SM. Toxin-mediated streptococcal and staphylococcal disease. J Am Acad Dermatol 1998;39:383-98.
[Google Scholar]
|
| 17. |
Martin JM, Green M. Group A streptococcus. Semin Pediatr Infect Dis 2006;17:140-8.
[Google Scholar]
|
| 18. |
Stock I. Streptococcus pyogenes--much more than the aetiological agent of scarlet fever. Med Monatsschr Pharm 2009;32:408-16.
[Google Scholar]
|
| 19. |
Feeney KT, Dowse GK, Keil AD, Mackaay C, McLellan D. Epidemiological features and control of an outbreak of scarlet fever in a Perth primary school. Commun Dis Intell 2005;29:386-90.
[Google Scholar]
|
| 20. |
Mc Donald M, Currie BJ, Carapetis JR. Acute rheumatic fever: A chink in the chain that links the heart to the throat? Lancet Infect Dis 2004;4:240-5.
[Google Scholar]
|
| 21. |
Sinclair D, Preziosi MP, Jacob John T, Greenwood B. The epidemiology of meningococcal disease in India. Trop Med Int Health 2010;15:1421-35.
[Google Scholar]
|
| 22. |
Majumdar T, Bhattacharya S, Barman D, Begum R. Laboratory confirmed outbreak of meningococcal infections in Tripura. Indian J Med Microbiol 2011;29:74-6.
[Google Scholar]
|
| 23. |
Deuren MV Brandtzaeg P, van der Meer JWM. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev 2000;13:144-66.
[Google Scholar]
|
| 24. |
Hackett SJ, Thomson APJ, Hart CA. Cytokines, chemokines and other effector molecules involved in meningococcal disease. J Med Microbiol 2001;50:847-59.
[Google Scholar]
|
| 25. |
Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Eng J Med 2001;344:1378-88.
[Google Scholar]
|
| 26. |
Sethi S, Sharma N, Kakkar N, Taneja J, Chatterjee SS, Banga SS, et al. Increasing trends of leptospirosis in northern India: A clinico-epidemiological study. PLoS Negl Trop Dis 2010;4:579.
[Google Scholar]
|
| 27. |
Sambasiva RR, Naveen G, Bhalla P, Agarwal SK. Leptospirosis in India and the Rest of the World. Braz J Infect Dis 2003;7:178-93.
[Google Scholar]
|
| 28. |
Sehgal SC. Epidemiological patterns in leptospirosis. Indian J Med Microbiol 2006;24:310-1.
[Google Scholar]
|
| 29. |
Swapna RN, Tuteja U, Nair L, Sudarshana J. Seroprevalence of leptospirosis of high risk groups in Calicut, North Kerala, India. Indian J Med Microbiol 2006;24:349-52.
[Google Scholar]
|
| 30. |
Basu D, Sarkar P, Chakraborty N, Chanda PR, Biswas S, Bera AB, et al. Leptospirosis and Weil's disease in eastern India. J Indian Med Assoc 2003;101:532, 534, 536.
[Google Scholar]
|
| 31. |
Chrispal A, Boorugu H, Gopinath KG, Prakash JA, Chandy S, Abraham OC, et al. Scrub typhus: An unrecognized threat in South India - clinical profile and predictors of mortality. Trop Doct 2010;40:129-33.
[Google Scholar]
|
| 32. |
Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, Mathai M, et al. Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci 2003;990:359-64.
[Google Scholar]
|
| 33. |
Vivekanandan M, Mani A, Priya YS, Singh AP, Jayakumar S, Purty S. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India 2010;58:24-8.
[Google Scholar]
|
| 34. |
Sharma VK, Sethuraman G, Kumar B. Cutaneous adverse drug reactions: Clinical pattern and causative agents: A 6 year series from Chandigarh, India. J Postgrad Med 2001;47:95-9.
[Google Scholar]
|
| 35. |
Sharma VK, Sethuraman G, Minz A. Stevens Johnson syndrome, toxic epidermal necrolysis and SJS-TEN overlap: A retrospective study of causative drugs and clinical outcome. Indian J Dermatol Venereol Leprol 2008;74:238-40.
[Google Scholar]
|
| 36. |
Sharma VK, Sethuraman G. Adverse cutaneous reactions to drugs: An overview. J Postgrad Med 1998;42:15-22.
[Google Scholar]
|
| 37. |
Guillaume JC, Roujeau JC, Revuz J, Pensol D, Touraine R. The culprit drugs in 87 cases of toxic epidermal necrolysis (Lyell's Syndrome). Arch Dermatol 1987;123:1166-70.
[Google Scholar]
|
| 38. |
D'Cruz DP. Systemic lupus erythematosus. BMJ 2006;332:890-4.
[Google Scholar]
|
| 39. |
Kim JG, Jung JY, Yoon BY, Hahn YS. Clinical Observations on Juvenile Rheumatoid Arthritis: I. Systemic Type. J Korean Rheum Assoc 1994;1:175-82.
[Google Scholar]
|
| 40. |
Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 1974;54:271-6.
[Google Scholar]
|
| 41. |
Yanagawa H, Nakamura Y, Yashiro M, Oki I, Hirata S, Zhang T, et al. Incidence survey of Kawasaki disease in 1997 and 1998 in Japan. Pediatrics 2001;107:33.
[Google Scholar]
|
| 42. |
Holman RC, Curns AT, Belay ED, Steiner CA, Schonberger LB. Kawasaki syndrome hospitalizations in the United States, 1997 and 2000. Pediatrics 2003;112:495-501.
[Google Scholar]
|
| 43. |
Park YW, Han JW, Park IS, Kim CH, Yun YS, Cha SH, et al. Epidemiologic picture of Kawasaki disease in Korea, 2000-2002. Pediatr Int 2005;47:382-7.
[Google Scholar]
|
| 44. |
Singh S. Kawasaki Disease: A clinical dilemma. Indian Pediatr 1999;36:871-5.
[Google Scholar]
|
| 45. |
Singh S, Kansra S. Kawasaki Disease. Natl Med J India 2005;18:20-4.
[Google Scholar]
|
| 46. |
Singh S, Bansal A, Gupta A, Manojkumar R, Mittal BR. Kawasaki Disease - A decade of experience from North India. Int Heart J 2005;46:679-89.
[Google Scholar]
|
| 47. |
Singh S, Gupta MK, Bansal A, Kumar RM, Mittal BR. A comparison of the clinical profile of Kawasaki disease in children from Northern India above and below 5 years of age. Clin Exp Rheumatol 2007;25:654-7.
[Google Scholar]
|
| 48. |
Tizard EJ, Hamilton-Ayres MJ. Henoch Schonlein purpura. Arch Dis Child Educ Pract Ed 2008;93:1-8.
[Google Scholar]
|
| 49. |
Saulsbury FT. Clinical update: Henoch-Schönlein purpura. Lancet 2007;369:976-8.
[Google Scholar]
|
| 50. |
Pachman LM, Cooke N. Juvenile dermatomyositis, A clinical and immunological study. J Pediatr 1980;96:226-34
[Google Scholar]
|
| 51. |
Dalakas MC, Hohfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971-82.
[Google Scholar]
|
| 52. |
Ozen S, Anton J, Arisoy N, Bakkaloglu A, Besbas N, Brogan P, et al. Juvenile polyarteritis: Results of a multicenter survey of 110 children. J Pediatr 2004;145:517-22.
[Google Scholar]
|
| 53. |
Feigin RD, Cherry JD. Cutaneous Manifestations of Systemic Infections. In: Fegin JD, Cherry, editors. Feigin and Cherry's Textbook of Pediatric Infectious Diseases. Vol. 2. Philadelphia: Saunders Elsevier; 2009. p. 755-80.
[Google Scholar]
|
| 54. |
Fölster-Holst R, Kreth HW. Viral exanthems in childhood - infectious (direct) exanthems. Part 2: Other viral exanthems. J Dtsch Dermatol Ges 2009;7:414-8.
[Google Scholar]
|
| 55. |
Ebell MH. Epstein-BarrVirus Infectious Mononucleosis. Am Fam Physician 2004;70:1279-87.
[Google Scholar]
|
| 56. |
Kessell I, Pollock TM, Edwards JMB, Woodroof M, Dodoo E, Melita DH, et al. Epstein-Barr virus infection in children entering a paediatric unit. J Infect 1980;2:269-74.
[Google Scholar]
|
| 57. |
Ranjit S, Kissoon N. Dengue hemorrhagic fever and shock syndromes. Pediatr Crit Care Med 2011;12:90-100.
[Google Scholar]
|
| 58. |
Rosario M, Capeding Z. Dengue update WHO 2009 guidelines, [homepage on the Internet] 2009. Available from: http://pidsphil.org/pdf/2010/10Lec-Dengue Update_WHO2009 Guideline REVISED.pdf. [cited 2011 Nov 4].
[Google Scholar]
|
| 59. |
Ravi V. Reemergence of chikungunya virus in India. Indian J Med Microbiol 2006;24:83-4.
[Google Scholar]
|
| 60. |
Mc Cornick JK. Toxic shock syndrome and bacterial superantigens: An Update. Annu Rev Microbiol 2001;55:77-104.
[Google Scholar]
|
| 61. |
Chi CY, Wang SM, Lin HC, Liu CC. A clinical and microbiological comparison of Staphylococcus aureus toxic shock and scalded skin syndromes in children. Clin Infect Dis 2006;42:181-5.
[Google Scholar]
|
| 62. |
Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet 2006;367:397-403.
[Google Scholar]
|
| 63. |
Abdel-Haq NM, Asmar BI, Abuhammour WM, Brown WJ. Yersinia enterocolitica infection in children. Pediatr Infect Dis J 2000;19:954-8.
[Google Scholar]
|
| 64. |
Karande S, Bhatt M, Kelkar A, Kulkarni M, De A, Varaiya A. An observational study to detect leptospirosis in Mumbai, India, 2000. Arch Dis Child 2003;88:1070-5.
[Google Scholar]
|
| 65. |
Rajajee S, Shankar J, Dhattatri L. Pediatric presentations of leptospirosis. Indian J Pediatr 2002;69:851-3.
[Google Scholar]
|
| 66. |
Steere AC. Lyme Disease. N Engl J Med 2001;345:115-25.
[Google Scholar]
|
| 67. |
Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis 2000;31:131-5
[Google Scholar]
|
| 68. |
Padbidri VS, Gupta NP. Rickettsiosis in India: A Review. J Indian Med Assoc 1978;71:104-7.
[Google Scholar]
|
| 69. |
Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)--A clinical reaction pattern. J Cutan Pathol 2001;28:113-9.
[Google Scholar]
|
| 70. |
Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, et al. The DRESS syndrome: A literature review. Am J Med 2011;124:588-97.
[Google Scholar]
|
| 71. |
Hazin R, Ibrahimi OA, Hazin MI, Kimyai-Asadi A. Stevens-Johnson syndrome: Pathogenesis, diagnosis, and management. Ann Med 2008;40:129-38.
[Google Scholar]
|
| 72. |
Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 1995;14;333:1600-7.
[Google Scholar]
|
| 73. |
Blume JE, Ali L, Ehrlich M. Drug eruptions. [homepage on the Internet] 2010. Available from http://emedicine.medscape.com/article/1049474. [cited 2011 Nov 3].
[Google Scholar]
|
| 74. |
Clark BM, Kotti GH, Shah AD, Conger NG. Severe serum sickness reaction to oral and intramuscular penicillin. Pharmacotherapy 2006;26:705-8.
[Google Scholar]
|
| 75. |
Committee on Rheumatic fever, Endocarditis, and Kawasaki Disease of the American Heart Association's Council on Cardiovascular Disease in the Young. Diagnostic guidelines for Kawasaki disease. Am J Dis Child 1990;144:1218-9.
[Google Scholar]
|
| 76. |
Mishra K, Choudhury J. Kawasaki disease: Is atypical more common than typical. Indian Pediatr 2006;43:453-4
[Google Scholar]
|
| 77. |
Rowley AH, Gonzalez-Crussi F, Gidding SS, Duffy CE, Shulman ST. Incomplete Kawasaki Disease with coronary artery involvement. J Pediatr 1987;110:409-13.
[Google Scholar]
|
| 78. |
Ramos MM, Tomashek KM, Arguello DF, Luxemburger C, Quinones L, Munoz JL. Early clinical features of dengue infection in Puerto Rico. Trans R Soc Trop Med Hyg 2009;103:878-84.
[Google Scholar]
|
| 79. |
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725.
[Google Scholar]
|
Fulltext Views
55,361
PDF downloads
10,921





