Translate this page into:
Fite-Faraco staining in combination with multiplex polymerase chain reaction: A new approach to leprosy diagnosis
2 Department of Dermatology, North Medical College, Siliguri, West Bengal, India
3 Department of Biochemistry, Apollo Gleangles Hospital, Kolkata, West Bengal, India
4 Department of Health, Govt. of West Bengal, West Bengal, India
5 Department of Pathology, JNM Medical College, Kalyani, West Bengal, India
6 Department of Dermatology, Medical College, Kolkata, West Bengal, India
Correspondence Address:
Basudev Bhattacharya
Department of Biochemistry, Institute of Post Graduate Medical Education and Research, Kolkata
India
| How to cite this article: Reja AH, Biswas N, Biswas S, Dasgupta S, Chowdhury IH, Banerjee S, Chakraborty T, Dutta PK, Bhattacharya B. Fite-Faraco staining in combination with multiplex polymerase chain reaction: A new approach to leprosy diagnosis. Indian J Dermatol Venereol Leprol 2013;79:693-700 |
Abstract
Background: Leprosy is not always an easy disease to diagnose, and patients can remain undiagnosed for longtime, not only at the peripheral clinics but also even at places with higher medical facilities, so, there is an urgent need for rapid and definitive modalities for leprosy diagnosis. This prospective study evaluates the ability of Fite-Faraco staining (FF staining) and multiplex polymerase chain reaction (PCR) over hematoxylin and eosin staining (H and E staining) and Ziehl-Neelsen staining (ZN staining). Aims: The aim of this perspective study is to evaluate the effectiveness of FF staining in combination with multiplex PCR for the early and rapid diagnosis of leprosy than any other coexisting diagnosis tool. Methods: Patients with new skin patches or nodules with or without evidence of nerve damage were selected for the study. Punch biopsy was collected according to standard procedures. Each biopsy sample was divided into two equal parts, one half was fixed in 4% (v/v) buffered neutral formalin and then accordingly embedded in paraffin. Sections were stained by three different methods: H and E staining for histopathological examination, ZN staining, and FF staining for detection of acid-fast bacilli (AFB). And the other part was subjected for DNA extraction and PCR was carried out by the obtained DNA sample. Results: H and E staining, ZN staining, FF staining, and PCR yield 58.2%, 50.9%, 60%, and 67.7% successful diagnosis of leprosy. The true diagnostic performances for these techniques were as follows: H and E staining - sensitivity 70.6%, positive predictive value (PPV) 81.9%, negative predictive value (NPV) 53.6%. For ZN staining - sensitivity 59.9%, PPV 69%, NPV 45.7%. For FF st aining - sensitivity 74.6%, PPV 85.9%, NPV 56.7%, and for PCR - sensitivity 87.8%, PPV 95.6%, NPV 71.2%. Conclusion: The combination of FF staining and PCR was shown to provide a rapid and definitive diagnosis in the majority of leprosy suspected cases with a higher positive likelihood ratio (+LR) of 7.76 and 2.716, respectively, than H and E staining of 2.244 and ZN staining of 1.378.Introduction
Leprosy has tormented the human civilization through the recorded history and has long been one of the world′s most stigmatized diseases, probably due to its mystery; fear of deformities and of contagion. Even today the stigma is still strong, despite our advanced knowledge about the disease and still remains an important health issue.
Leprosy is not always an easy disease to diagnose, and patients can remain undiagnosed for a longtime, not only at the peripheral clinics but also even at places with higher medical facilities. So, early diagnosis is of fundamental importance to all aspects of leprosy including epidemiology, case management, and the prevention of deformity and disability.
Mycobacterium leprae (M. leprae) cannot be cultivated in vitro, and so the classical bacteriological methods to identify pathogenic bacteria cannot be applied for the diagnosis of leprosy. The diagnosis of leprosy has been performed based on clinical criteria (anesthetic skin lesions; enlarged/thickened peripheral nerves) and the presence of acid-fast bacilli (AFB) from tissue smears or tissue section stained by modified Ziehl-Neelsen staining (ZN staining). Laboratory diagnosis of leprosy is generally made by microscopic and histopathological examination. [1] Slit skin smears (SSSs), taken to detect intradermal bacilli, traditionally represents, one of the cardinal signs of the disease and when positive for M. leprae have very high specificity but its sensitivity is extremely low because smear positive patients never contribute higher percentage of the total number of patients, on the contrary 70% of leprosy patients are smear negative. So low degree of positivity combined with low reliability of the technique may make reliance on them more difficult. Biopsy to study histopathology, immunohistopathology, and culture in mouse foot pad are very helpful tests but are available only in few centers. Histopathology continues to be regarded as the gold standard for the diagnosis, particularly in early stage of leprosy. It also provides information on the nature of host response by which one can predict the likely course of the disease and the likely response to therapy. Literature reveals that the histopathological conformation rate in several studies varies from 29% to 58% in early stages of leprosy and clinically suspected leprosy. Job-Chacko modified Fite-Faraco (FF) staining for M. leprae enables the better chances of detection of the bacteria in the granuloma of early leprosy. [2]
Nonpolymerase chain reaction (PCR) based detection of M. leprae DNA requires at least 10 4 organisms in order to obtain reliable and reproducible results. [3] Therefore, these methods are not routinely used as a diagnostic tool to detect M. leprae, particularly in patients with indeterminate type and the tuberculoid end of the leprosy spectrum where AFB are generally rare or virtually absent.
New molecular biology methods exploiting nucleic acids technology have been developed as reliable and sensitive diagnostic tools for identification of pathogens in many infectious diseases. With the specific PCR amplification of M. leprae DNA it becomes much easier for the detection and diagnosis of leprosy, due to its sensitive, specific, and rapid detection of microorganisms in clinical specimens especially in the paucibacillary (PB) entity of leprosy. [4]
The aim of this study is to evaluate and compare the effectiveness of tissue section stained with three different stains like hematoxylin and eosin staining (H and E staining), FF staining, and modified ZN staining in combination with PCR in different entities of leprosy for rapid, early and possible accurate diagnosis.
Methods
Leprosy patients were selected following World Health Organization (WHO) guidelines and were classified according to the Ridley-Jopling Classification. [5]
Collection of punch biopsies
Samples from untreated patients were collected after obtaining duly signed informed consent forms and institutional ethical clearance. Patients with new skin patches or nodules with or without evidence of nerve damage were selected for the study. Samples for punch biopsy of approximately 4 mm size were collected according to standard procedures. [1] A portion of biopsy samples from each patient was fixed in 4% (v/v) buffered neutral formalin and then dehydrated in a graded series of ethanol and embedded in paraffin. Sections were stained by three different methods: H and E staining for histopathological examination, ZN staining, and FF staining for detection of AFB and other portion had been stored at −20°C until used for PCR.
H and E staining for tissue sections
H and E staining for paraffin section was done by deparaffinizing in xylene followed by dehydration through graded alcohols and then stained in Harris hematoxylin for 15 min followed by washing under running tap water and differentiation in 1% acid alcohol and were counterstained with 1% eosin for 1 min followed by washing and blotting and finally mounted with DPX. [6],[7]
Modified ZN staining for tissue sections
Modified ZN staining was done for the tissue sections briefly. Section were deparaffinized, stained in boiling/hot carbol fuchsin for 15 min, washed and decolorized in 25% H 2 SO 4 , washed and counter stained with 1% methylene blue. Slides were air dried and mounted with DPX. [8]
Fite-Faraco staining for tissue sections
Sections were deparaffinized with two changes of 12 min each of xylene-peanut oil and stained in carbol fuchsin stain for 30 min, washed in running tap water, decolorized by 5% H 2 SO 4 differentiated in 25% ethanol followed by washing again under running tap water. Slides were counterstained with Harris hematoxylin for 3 min. Excess hematoxylin was washed off, blotted, and kept for a few minutes for air dry and finally mounted with DPX. [2]
Extraction of DNA from the biopsy samples and multiplex polymerase chain reaction
Skin Biopsies were collected from study subjects and genomic DNA was isolated by proteinase-K digestion and a standard phenol chloroform method. [9]
Multiplex PCR
A multiplex PCR for the rapid diagnosis of M. leprae in patients has been developed in our laboratory based on the following primers:
- The repetitive sequence of the M. leprae DNA, reported to be very specific to M. leprae and not present in 20 other mycobacterial species other than M. leprae.[10]
- A region flanking entire 21TTC repeat sequence specific for multibacillary (MB) leprosy designed by Shin et al. The specificity and sensitivity of the primers: LR1 and LR2 and TTC-A and TTC-B had been already established in earlier studies. [11] PCR was performed by Multiplex PCR technique as described earlier. [9] Briefly, 100 ng genomic DNA was amplified with DNA polymerase (Ampli Taq Gold; Applied Biosystems, Inc. [ABI], Foster City, CA) in a PCR reaction mixtures, containing 1 × PCR buffer (Applied Biosystems), 2 mM MgCl2, 0.25 mM each dNTP, 20 picomoles generic antisense primers, and 20 picomoles of generic sense primers. The primer sequences, primer annealing temperature (Ta°), and PCR product sizes are shown in [Table - 1]. The PCR reactions were performed in the following conditions: 95°C 4 min, followed by 35 cycles of 95°C for 1 min, Ta° for the internal control as shown in [Table - 1] for 1 min, 72°C for 1 min, and then 72°C for 10 min for the final extension. The amplified products were separated by electrophoresis on 2% agarose gel stained with 0.5 mg/ml ethidium bromide and visualized and photographed under a UV transilluminator.
 Table 1: Primers and Tm values for PCR
Table 1: Primers and Tm values for PCR
Statistical analysis
All statistical analysis is done by online diagnostic test calculator. ( http://ktclearinghouse.ca/cebm/practise/ca/calculators/statscalc ). Canadian Institute of Health Research (CIHR) 2000-2012. [12],[13],[14],[15],[16],[17]
Results
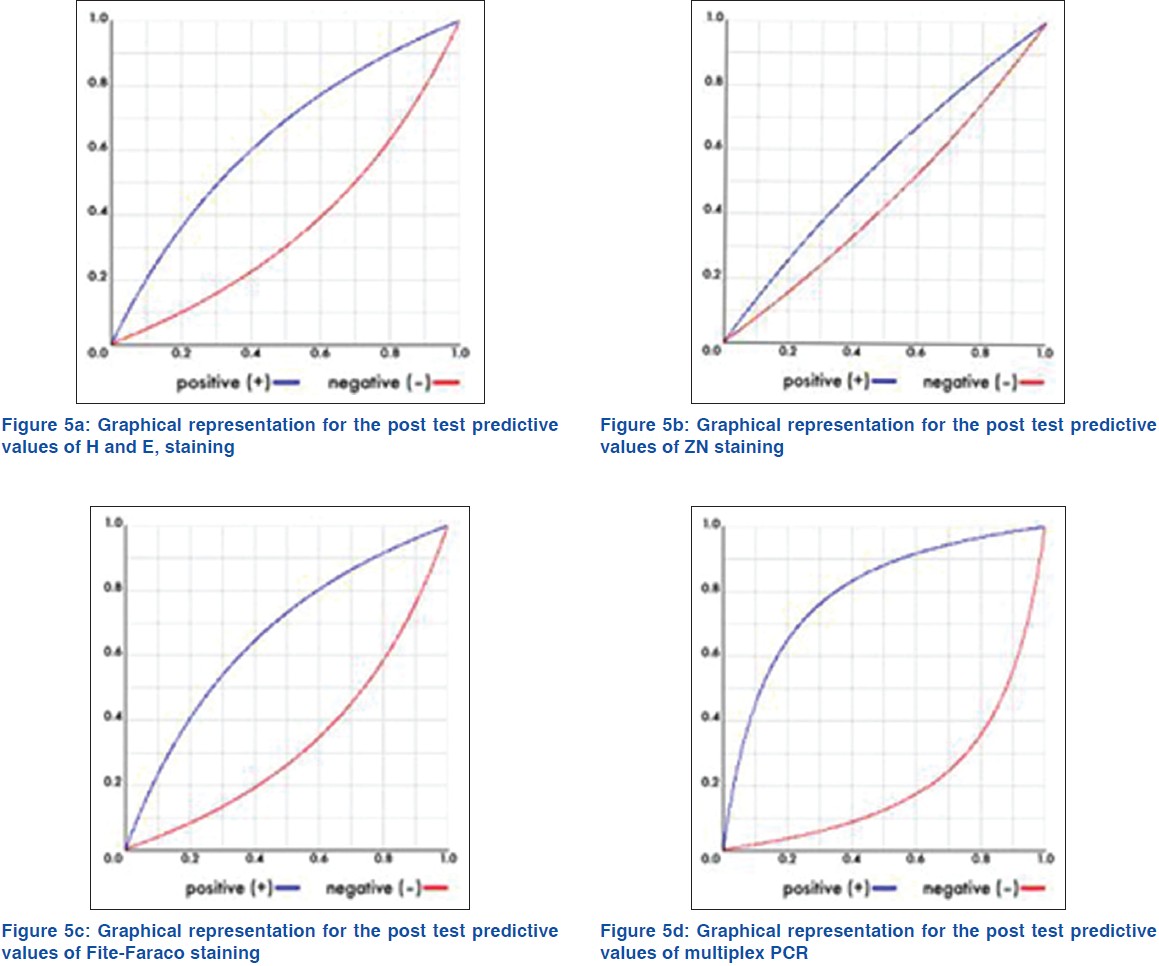
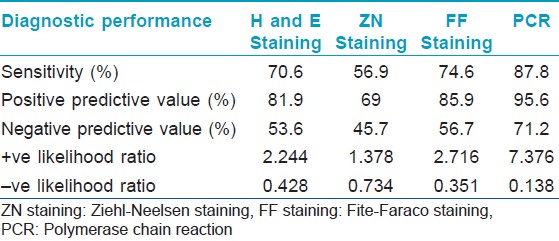
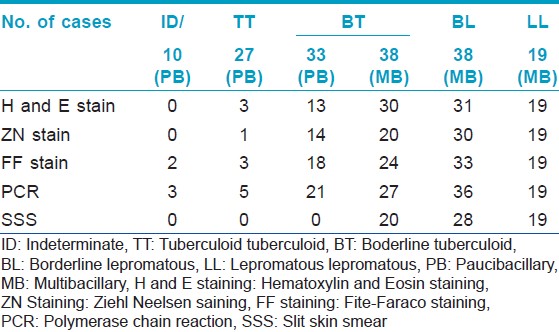
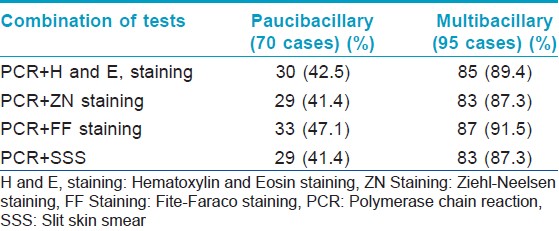
One hundred and sixty five (n = 165) patients clinically diagnosed as leprosy cases were enrolled in this study out of which 10, 27, 71, 38, and 19 cases were diagnosed as indeterminate, TT, BT, BL, and LL, respectively. Punch biopsies, taken from these patients were processed for histopathological studies [Figure - 1], [Figure - 2] and [Figure - 3]. Considering clinical diagnosis as a reference the concordance of different techniques were as follows: H and E staining suggested 96 cases (58.2%) as positive with an indirect evidence of M. leprae infection as in [Table - 2]. Modified ZN staining showed total 84 cases (50.9%) positive for the presence of AFB in the tissue section while FF staining and PCR [Figure - 4] showed 99 (60%) and 111 (67.3%) positive cases, respectively, as shown in [Table - 2]. The "true" diagnostic performance of the various techniques is shown in [Table - 3] and [Table - 4] with the graphical representation in [Figure - 5]a-d.
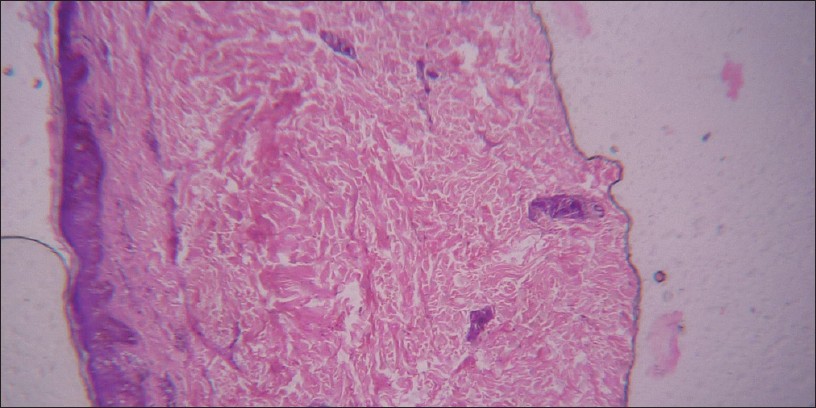 |
| Figure 1: H and E, staining for tissue section showing increased spaces between dermis and epidermis depicting an indirect evidence for M. leprae infection (H and E stain viewed under ×100 immersion oil) |
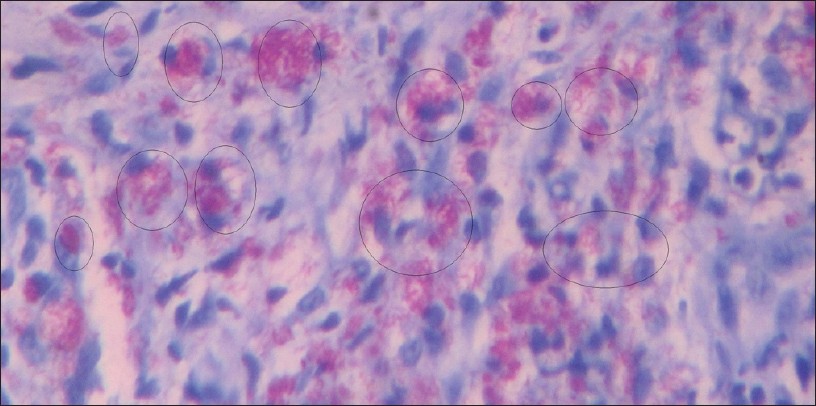 |
| Figure 2: Ziehl– Neelsen staining for tissue section (Carbol Fuchsin stained counter stained by methylene blue viewed under ×100 immersion oil). Circles showing granuloma |
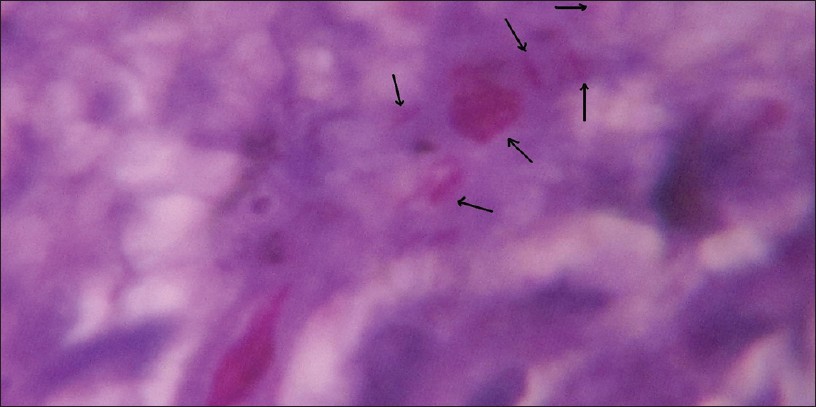 |
| Figure 3: Fite-Faraco staining for tissue section (Carbol Fuchsin stain counter stain with hematoxylin viewed under ×100 immersion oil). Arrows showing M. leprae bacilli |
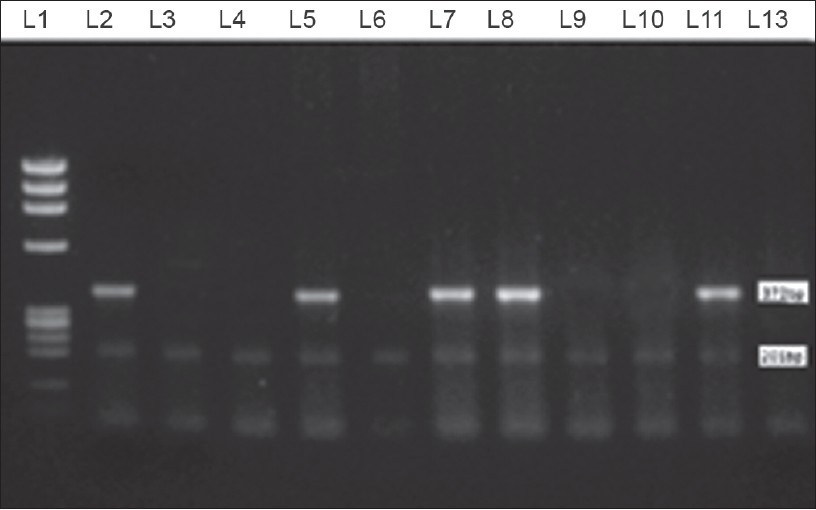 |
| Figure 4: Multiplex PCR showing Lane1: PhiX174 DNA Marker, Lane 2, 5, 7, 8: shows positive bands for both 372bp and 201 bp. Lane 3, 4, 6, 9, 10: Shows positive band for 201 bp only. Lane 11: Positive control (Thai 53 strain) Lane 12: Negative control |
 |
| Figure 5: |

Discussion
The more subtle issue in an early diagnosis of leprosy is the designation of a true positive result. It became very difficult to diagnose leprosy by the clinicians at the early stage when it would often have offered the opportunity to treat disease. Hence, it seems important for the clinicians to have an ideal diagnostic tool that would be simple and with high specificity and sensitivity. Combining various tests may improve the precision of a diagnostic procedure in leprosy as using the ′OR′ connector sensitivity is increased at the expense of specificity, whereas using the ′AND′ connector (a combination of two or more signs must be present for the diagnosis) increases specificity at the expense of sensitivity. The sensitivity and specificity of a test can be determined only by comparison with another reliable test, that is, a so-called ′gold standard′, if it exist keeping clinical diagnosis as reference. The "gold standard" for the diagnosis of leprosy is a full thickness skin biopsy sample obtained from the advancing margin of an active lesion, fixed in neutral buffered formalin, embedded in paraffin, and examined by an experienced pathologist. The primary characteristics to be recognized are histological patterns of the host response in H and E stained sections, the involvement of cutaneous nerves, and the identification of AFB within nerves using the ZN staining. [18]
Presently, histopathology continues to be regarded as the gold standard for the diagnosis, with its limitations, particularly in early stage of leprosy. The histopathology of infectious diseases, that is, direct microscopic visualization of tissue samples for identification of the infectious agent, is particularly useful when cultures cannot be made or the infectious agent is slow growing or fastidious. [1],[19] When considered with relevant clinical history, the histological features seen in tissue biopsies may provide sufficient information to correctly identify a particular type of organism. [20],[21]
In the present study, H and E staining as in [Figure - 1], identified 58.2% of the 165 of cases as positive with indirect evidence as presented in [Table - 2], among which 22.8% of 42.6% PB cases and 84.2% of 57.6% MB cases as in [Table - 5], with sensitivity of 70.6%. Also the positive predictive value (PPV) and the negative predictive value (NPV) for this test were 81.9% and 53.6%, respectively, as shown in [Table - 3] and graphically shown in [Figure - 5]a. Furthermore, 1 out of 44 PB cases and 3 out of 10 MB cases were found positive, which were found negative in PCR as in [Table - 6]. Hence combination of H and E staining and PCR yielded 42.5% of the total PB cases and 89.4% of the total MB cases as shown in [Table - 7].


Modified ZN staining as in [Figure - 2] showed 50.9% of 165 cases as positive for the presence of M. leprae in the tissue section as presented in [Table - 2] among which 21.4% of 42.6% PB cases and 72.6% of 57.6% MB cases as shown in [Table - 5] with sensitivity of 56.7% and the PPV and NPV for this test were 69% and 45.7%, respectively, as shown in [Table - 3] and graphically presented in [Figure - 5]b. Furthermore, 1 out of 10 MB cases were found positive, which were found negative in PCR as in [Table - 6]. Hence, combination of modified ZN stain and PCR yielded 41.4% of the total PB cases and 87.3% of the total MB cases as shown in [Table - 7].
The FF staining for leprosy bacilli is similar to the modified ZN staining, but the paraffin wax is removed from the sections with two changes of xylene with one part of groundnut oil or cottonseed oil. [2] Mycobacterial cell walls contain a waxy substance composed of mycolic acids. These are ί-hydroxyl carboxylic acids with chain lengths of up to 90 carbon atoms. The property of acid fastness is related to the carbon chain length of the mycolic acid. Deparaffinizing using peanut oil/xylene mixture helps to protect the more delicate waxy coat of the organisms and the residual oil in the sections helps prevent shrinkage. Also the modified method uses hematoxylin as the counter stain in place of methylene blue. The leprosy bacillus is more easily decolorized than the tubercle bacillus, and differentiation must be carefully controlled therefore alcohol is removed from the hydrating and dehydrating steps and 5% sulphuric acid is used as a decolorizer in place of acid-alcohol solution. As hematoxylin is used as the counter stain in the FF staining, it demonstrates the nucleus and cytoplasmic inclusions. A faint residual red color in the tissues is especially important, and sections that do not show this may be unreliable for the exclusion of leprosy, therefore the cells and the tissue structures are clearly identified in relation to the AFB. Finding AFB in any one of the following sites such as the sub-epidermal region of the skin, inside Schwann cells, endothelial cells will confirm the diagnosis of leprosy.
In the present study, FF staining of tissue section from skin biopsies as shown in [Figure - 3], showed 60% of the 165 cases as positive for the presence of M. leprae in the tissue sections as shown in [Table - 2] among which 33.5% of 42.6% PB cases and 80% of 57.6% MB cases as in [Table - 5]. This test showed sensitivity of 74.6% and PPV of 85.9% and NPV of 56.7%, respectively, as shown in [Table - 3] and graphically shown in [Figure - 5]c. Also, by this test 4 out of 44 PB cases and 5 out of 10 MB cases were found positive, which were found negative in PCR as shown in [Table - 6]. Hence combination of FF staining and PCR yielded 47.1% of the total PB cases and 91.5% of the total MB cases as shown in [Table - 7].
M. leprae is not cultivable in vitro, and lack of growth on standard mycobacterial isolation media could be considered as one laboratory criterion to differentiate this organism from other mycobacterial pathogens, at the same time definitive identification of M. leprae is problematic and eventually this problem is confounded today by the increased prevalence of other mycobacterial infections of the skin. The major achievements for laboratory diagnosis of Hansen′s disease for the past 20 years were the development of methods for the extraction, amplification, and identification of M. leprae DNA in clinical specimens using PCR and other molecular techniques.
These assays have been based primarily on the amplification of M. leprae specific sequences using PCR and identification of the M. leprae DNA fragment. Many different M. leprae genes have been utilized in the development of PCR assays for detection of M. leprae in clinical specimens. Rapid molecular-type assays have been developed for detection of M. leprae directly from patient specimens using available genetic data. [22]
In the present study, 67.3% cases of 165 cases as shown in [Table - 2] were found positive for the presence of M. leprae DNA in the tissue section when subjected to PCR as shown in [Figure - 4], among which 41.4% of 42.6% PB cases and 86.3% of 57.6% MB cases as shown in [Table - 5]. This test showed sensitivity of 87.8% and with PPV of 95.6% and NPV of 71.2% as shown in [Table - 3] and graphically shown in [Figure - 5]d.
In addition of these test SSS was also done for the 165 cases out of which 40.6% cases were found positive for the presence of M. leprae under the microscope as shown in [Table - 2]. Further SSS in combination of PCR, showed 41.4% of the total PB cases and 87.3% of the total MB cases as shown in [Table - 7].
A widely held belief is that one should diagnose a disease first with a sensitive test and then follow up the occurrence of positive result with a specific test for best performance. [23] The logic is that if the first test determines which patients are to undergo a second test, then the first test should be more sensitive of the two to ensure that the disease has not been missed. Somewhat surprisingly, the order in which the tests are performed does not affect the combination of sensitivity and specificity. However, it does not affect the overall cast. Hence, on the basis of extensive assessment of these tests in this study we diagnosed the patient first with FF staining (sensitivity 74.6%) followed by multiplex PCR (specificity 100%) and found that the combination of these test yields 47.1% of the total PB cases and 91.5% of the total MB cases as shown in [Table - 7], which is higher as compared with H and E staining (42.5% PB and 89.4% MB) and ZN staining (41.4% PB and 87.3% MB) as shown in [Table - 7]. As also, the positive likelihood ratio (+LR) for PCR (7.376) and FF stain (2.716) is quite higher than that of H and E staining (2.244) and ZN staining (1.378) as shown in [Table - 3]. Thus, the combination of PCR and FF staining can be considered as better diagnostic tool than the combination of H and E, staining and ZN staining.
Conclusion
Based on the above discussion it may be said that in the field of laboratory-based diagnosis of leprosy cases, FF staining in combination with multiplex-PCR is much more reliable and efficient technique than any other coexisting diagnostic techniques for the early and rapid diagnosis of leprosy. However, proper assessment of leprosy cases by these techniques will enlighten the future path of reliable and easy diagnosis of leprosy specially will reinforce the leprosy elimination process.
| 1. |
Watts JC, Chandler FW. The surgical pathologist's role in the diagnosis of infectious diseases. J Histotechnol 1995;18:191-3.
[Google Scholar]
|
| 2. |
Job CK, Chako CJ. Modification of Fite's stain for demonostration of M.leprae in tissue sections. Indian J Lepr 1986;58:17-8.
M.leprae in tissue sections. Indian J Lepr 1986;58:17-8.'>[Google Scholar]
|
| 3. |
Fujiwara J, Oishi M. Direct probing: Covalent attachment of probe DNA to double stranded target DNA. Nucleic Acids Res 1998;26:5728-33.
[Google Scholar]
|
| 4. |
Torres P, Camarena JJ, Gomez JR, Noguira JM, Gimeno V, Navarro JC, et al. A Comparison of PCR mediated amplification of DNA and the classical methods for detection of Mycobacterium leprae in different types of clinical samples in leprosy patients and contacts. Lepr Rev 2003;74:18-30.
[Google Scholar]
|
| 5. |
Ridley DS, Jopling WH. Classification of leprosy according to immunity- A five group system. Int J Lepr 1996; 34:255-72.
[Google Scholar]
|
| 6. |
Lillie RD, Fullmer HM. Histopathologic Technic and Practical Histochemistry, 3 rd ed.. New York: McGraw-Hill Book Co; 1976. p. 972.
[Google Scholar]
|
| 7. |
Mayer P. Mitt. Zool. Stn. 12 th ed.. Neapel; 1896. p. 303.
[Google Scholar]
|
| 8. |
Allen JL. A modified Ziehl Neelsen for mycobacteria. Med Lab Sci 1992;49:99-102.
[Google Scholar]
|
| 9. |
Banerjee S, Sarkar K, Gupta S, Mahapatra PS, Gupta S, Guha S, et al. Multiplex PCR technique could be an alternative approach for early detection of leprosy among close contacts: A pilot study from India. BMC Infect Dis 2010;10:252.
[Google Scholar]
|
| 10. |
Yoon KH, Cho SN, Lee MK, Abalos RM, Cellona RV, Fajardo TT, et al. Evaaluation of polymerase chain reaction amplification of Mycobacterium leprae specific sequence in biopsy specimens from leprosy patients. J Clin Microbiol 1993;31:895-9.
[Google Scholar]
|
| 11. |
Shin YC, Lee H, Walsh GP, Joo-Deuk K, Cho SN. Varable numbers of TTC repeats in Mycobacterium leprae DNA from leprosy patients and use in strain differentiation. J Clin Microbiol 2000;38:4535-8.
[Google Scholar]
|
| 12. |
Gardner IA, Greiner M. Receiver-operating characteristic curves and likelihood ratios: Improvements over traditional methods for the evaluation and application of veterinary clinical pathology tests. Vet Clin Pathol 2006;35:8-17.
[Google Scholar]
|
| 13. |
Griner PF, Mayewski RJ, Mushlin AI, Greenland P. Selection and interpretation of diagnostic tests and procedures. Ann Intern Med 1981;94:555-600.
[Google Scholar]
|
| 14. |
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29-36.
[Google Scholar]
|
| 15. |
Metz CE. Basic principles of ROC analysis. Semin Nucl Med 1978;8:283-98.
[Google Scholar]
|
| 16. |
Zhou XH, Obuchowski NA, McClish DK. Statistical methods in diagnostic medicine. 16 th ed. New York: Wiley and Sons Interscience; 2002. p. 437.
[Google Scholar]
|
| 17. |
Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem 1993;39:561-77.
[Google Scholar]
|
| 18. |
Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev 2006;19:338-81.
[Google Scholar]
|
| 19. |
Renshaw AA. The relative sensitivity of special stains and culture in open lung biopsies. Am J Clin Pathol 1994;102:736-40.
[Google Scholar]
|
| 20. |
Procop GW, Wilson M. Infectious disease pathology. Clin Infect Dis 2001;32:1589-601.
[Google Scholar]
|
| 21. |
Woods GL, Walker DH. Detection of infection or infectious agents by the use of cytologic and histologic stains. Clin Microbiol Rev 1996;9:382-404.
[Google Scholar]
|
| 22. |
Katoch VM. Molecular techniques for leprosy: Present applications and future perspectives. Indian J Lepr 1999;71:45-59.
[Google Scholar]
|
| 23. |
Watts NB. Medical relevance of laboratory test: A clinical perspective. Arch Pathol Lab Med 1988;112:379-82.
[Google Scholar]
|
Fulltext Views
19,309
PDF downloads
4,421





