Translate this page into:
For the love of color: Plant colors and the dermatologist
2 Department of Dermatology, Venereology and Leprosy, Sri Manakula Vinayagar Medical College and Hospital, Pondicherry University, Puducherry, India
Correspondence Address:
Hima Gopinath
Department of Dermatology, All India Institute of Medical Sciences, Mangalagiri - 522 503, Guntur District, Andhra Pradesh
India
| How to cite this article: Gopinath H, Karthikeyan K, Meghana V. For the love of color: Plant colors and the dermatologist. Indian J Dermatol Venereol Leprol 2020;86:622-629 |
Abstract
Humans have been anointing their skin with natural colorants since antiquity. Before the advent of modern cosmetics, tattoos and hair dyes, the spectacular colors in plants served as a palette for humanity's fascination with color. Skin, hair, nails, teeth and clothing have been altered with botanical colorants for centuries. Understanding the relevance of botanical colorants is an important part of cultural competency. Substitution or adulteration of plant colorants with synthetic colorants has played a role in varied dermatoses (eg. black henna, kumkum, and Holi dermatoses). Safety concerns over synthetic colorants have led to a resurgence of natural colorants. However, some plant colorants have produced adverse reactions. Plant colorants have also played an integral role in medicine. Ingested plant colorants are an indispensable part of our diet, playing crucial roles in the maintenance of health and prevention of disease. Excessive intake of some pigments can alter skin color (carotenoderma, lycopenemia, and the golden tan of canthaxanthin). We have relied on the colors of hematoxylin and alizarin red, derived from the logwood tree and madder roots, respectively, to study and diagnose disease in pathology. We briefly review the uses, cultural relevance, and adverse effects of the common botanical colorants on the skin, hair, and mucosa. We also describe their relevance in our diet, and in the diagnosis and description of dermatological diseases.
Introduction
From palaeolithic times, humans have enriched their appearance with various body paints derived from nature.[1] Plants, minerals and insects have been used to color skin, hair, nails, and clothing for aesthetic, ceremonial, military and religious reasons.[2] The major botanical colorant groups such as chlorophylls, carotenoids, flavonoids, and betalains, along with several other minor colorant groups, provide an endless array of colors that has inspired art and intrigued medicine.[3] We briefly review the decorative, dietary, diagnostic and descriptive aspects of botanical colorants in dermatology.
Henna
Henna is obtained from the plant Lawsonia inermis [Figure - 1], an ancient medicinal shrub. It has been used by Egyptians as seen in the mummies to color hair and nails, and in the Orient to color skin, hair and nails. It was brought to India in the 12th century by the Mughals from Persia. It is traditionally used to color hands and feet (Mehndi) before weddings and other celebrations.[4] Muslims and Orthodox Jews have used it to color natural textiles. Surgeons in India have used it as a pre-operative skin marker.[5] It has also been used to camouflage vitiligo, skin scars and brittle, dystrophic nails.[6]
 |
| Figure 1: Henna leaves and henna powder prepared from dried henna leaves |
The active coloring agent is lawsone, 2-hydroxy-1,4-naphthoquinone, which is present in concentrations lower than two percent in natural henna preparations. It binds to keratin to produce the reddish-brown (rust-brown) color of 'red henna'. The dried leaves (green-gray in color) are mixed with water or oil and allowed to dry on the skin for around 30 min to 6 hours. A longer duration of exposure produces a darker color.[5] A number of agents are added to henna to darken the color [Figure - 2] or increase its appeal. These include synthetic coloring agents such as paraphenylenediamine and diaminotoluenes, coffee, black tea, turpentine, lemon juice, tamarind, eucalyptus oil, mustard oil, clove oil, fenugreek seed and fresh urine of animals such as yak and camels.[4]
 |
| Figure 2: Orange colour and brownish-black colour produced by a mixture of henna and tamarind left overnight over hands |
Henna is also the most popular botanical hair dye. Lawsone has been demonstrated in the cuticle of Ramses II, a king of Egypt in the 13th century before common era.[7] It provides an auburn shade [Figure - 3]. Other plants have been added to provide a range of colors: a blue-black color with 'henna- reng' (a combination of henna and indigo leaves), blackish hues with the addition of indigo leaves and walnut leaves, golden hues with Turkey rhubarb (Rheum palmatum) and chamomile, and a coppery hue with onion skins (Allium cepa).[8],[9]
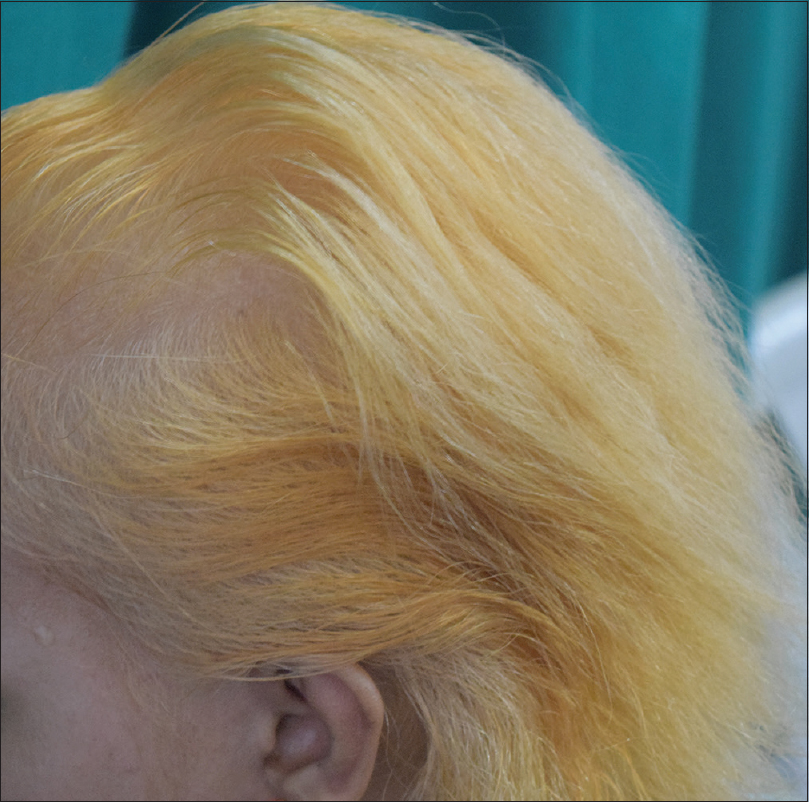 |
| Figure 3: Regrowing white hair of alopecia totalis camouflaged with henna |
Sensitization to natural henna is rare despite its extensive use. There have been a few reports of allergic contact dermatitis and ectopic dermatitis with natural henna. The allergens may be lawsone or some other unidentified allergens. IgE mediated reactions may occur, particularly in hairdressers with symptoms such as conjunctivitis, rhinitis, cough, facial edema and urticaria. Lawsone can cause oxidative hemolysis and may cause life-threatening hemolysis in children with glucose-6 phosphate dehydrogenase deficiency.[5]
The agents added to henna paste often contribute to the adverse reactions associated with temporary henna tattoos. Paraphenylene diamine is the most important sensitizer in 'black henna'.[4] Black henna is often marketed as a safe, cheap and natural hair dye or as instant temporary tattoos. These pseudotattoos are a popular alternative to the painful permanent tattoos done with needles. However, they can cause local and life-threatening systemic complications. These are listed in [Table - 1].[5],[10],[11]

Jagua Tattoos
The jagua fruit (Genipa americana) is becoming increasingly popular for producing temporary blue-black tattoos. Genipin is the colorless active ingredient that turns blue-black on exposure to skin. Genipa americana is native to the Amazon basin and has been used by indigenous people to color skin, hair and clothes. Gardenia jasminoides, used in traditional Chinese medicine, is another source of genipin. Genipin is being marketed as a natural 'paraphenylene diamine free' substitute for synthetic tattoos and black henna. It has an affinity for proteins and amino acids. It can cause allergic contact dermatitis. It is also used as a finger-print reagent and a food coloring agent (Jagua blue or Gardenia blue).[12],[13]
Other Botanical Skin and Hair Colorants
Turmeric containing yellow curcuminoids, is an ancient cosmetic that imparts a golden hue to the skin. Women in India traditionally apply turmeric daily or during religious ceremonies. It is anti-inflammatory, anti-oxidant and improves the appearance of photodamaged skin. It provides natural yellow color to skincare creams. Adverse effects include contact urticaria, allergic contact dermatitis and pigmented cosmetic contact dermatitis.[14]
Kumkum and red sandalwood (Pterocarpus santalinus) are common red colorants while sandalwood (Santalum album) is a yellow one. They are traditionally applied on the forehead by Hindus in India for socioreligious reasons (personal observation). Kumkum is traditionally prepared at home from slaked time and turmeric and applied on the forehead or hair-parting. However, it is now commercially produced with several compounds which may include coal tar dyes, toluidine red, erythrosine, lithol red calcium salt, lead oxide, fragrances, groundnut oil, tragacanth gum and parabens, canaga oil, turmeric and sandalwood. It can result in allergic contact dermatitis, pigmented contact dermatitis and chemical leukoderma.[15]
Several plant colors such as annatto (red from Bixa orellana), indigo (blue from Isatis tinctoria or Indigofera species), safflower (red from Carthamus tinctorius), gamboge (yellow from Garcinia xanthochymus) and soot have been used in body painting or tattooing in ancient tribes and cultures. In Japan, the geisha has white face due to powdered rice flour and lips red due to safflower.[16] Betel quid has long-lasting red tannins that have been used to create a natural red lipstick.[17] Thus, modern cosmetics and tattoos have their origins in the ancient practices of anointing the skin with natural colorants. They are being re-explored for safer cosmetics and tattoos. They may have additional medicinal effects. However, plant products may cause allergic contact dermatitis, phytophotodermatitis, and alter pigmentation by enhancing or suppressing melanogenesis.[18]
Several botanical colorants, in addition to henna, have been explored in the ancient art of hair coloring. Roman women used walnut extracts to color their hair.[6] Acute irritant contact dermatitis has been reported with walnut extracts (juglone).[19] Chamomile contains the flavonoid apigenin, and provides a dull yellow hue to fair hair. Curcumin may be used to obtain yellow to orange hues [Figure - 4]. Hibiscus (Hibiscus sabdariffa) produces reddish hues due to the presence of anthocyanins.[9] Black marking nut juice has also has been used in indigenous hair dyes resulting in severe irritant and allergic reactions. Dermatitis may spread to other areas through the hands.[20] Home-based and traditional preparations used in India to darken hair, and prevent or cover canities include combinations containing henna, indigo (Indigofera tinctoria), bhringaraj (Eclipta alba), amla (Emblica officinalis), catechu (Acacia catechu) black tea, curry leaves, fenugreek seeds, beetroot and coffee (personal observation). Tannins and metallic mordants (including toxic heavy metals) are often added to botanical dyes to bind the dye to the hair. Tea has been used as a natural source of tannins. However, walnut extracts (juglone or 5-hydroxy-1,4-naphthoquinone) and henna (lawsone or 2-hydroxy-1,4-napthoquinone) bind directly to hair without the need for a mordant.[21] Natural hair dyes may have additional problems with stability, oxidation and discoloration, pH color shift, fading, reproducibility, and limitations in the range of colors.[9] Natural hair colorants may be a source of artifacts on trichoscopy.[22]
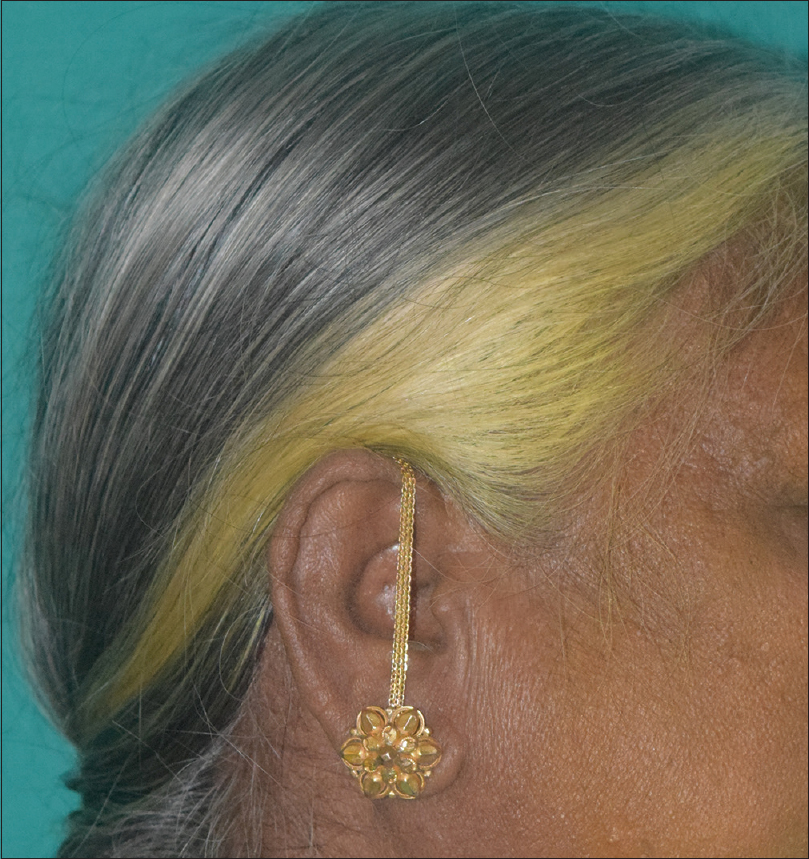 |
| Figure 4: Yellow discolouration of hair with the use of turmeric |
Holi, the festival of colors, is a popular Hindu festival in India. It heralds the arrival of spring and is a celebration of new life and colors. Colored powders or liquids are applied or splashed on family, friends and strangers.[23] Traditional colorants were obtained from botanical sources such as yellow from turmeric (Curcuma longa), green from neem (Azadirachta indica) and red from palash flowers (Butea monosperma).[24] Botanical colorants were presumed to be medicinal in Ayurveda. Cheap and unregulated synthetic colorants (such as malachite green, rhodamine, gentian violet, auramine O, lead oxide, copper sulphate, mercury sulphite and chromium iodide used along with silica and mica) have replaced the traditional botanical colorants. This has resulted in an annual spate of 'Holi dermatoses' such as, allergic and irritant contact dermatitis, photosensitive eruptions, secondary pyoderma, methemoglobinemia (with aniline dyes), exacerbations of pre-existing dermatoses such as acne, eczema and paronychia, and ocular complications that range from mild chemical conjunctivitis, corneal keratopathy to infected corneal ulcers.[23],[25],[26] Natural colorants for Holi may be made at home [Figure - 5], albeit with a little effort and added expense. However, there are possibilities of allergic contact dermatitis, bacterial and fungal contamination, and pesticide residues leading to health issues.
 |
| Figure 5: Natural holi colours being prepared at home from beetroot, chrysanthemum, coffee, chrysanthemum, hibiscus, turmeric and neem (clockwise from arrow and centre respectively) |
Oral Cavity
Betel
Betel chewing is a 2000-year-old practice and is popular in South Asia, Southeast Asia and South-Pacific. Betel is a pharmacologically addictive stimulant that is ingrained into various cultural practices and ceremonies.[27] It has been used in traditional Ayurvedic medicine for various disorders (including as a laxative, carminative). It is the fourth most commonly consumed drug in the world behind caffeine, ethanol and nicotine.
The fruit or nut of the Areca palm (Areca catechu), the leaves of betel pepper (Piper betle) and lime (calcium hydroxide) are chewed together to produce the 'blood-red' betel juice (betel quid or paan). It may be mixed with tobacco or with aromatic spices such as cloves, cardamom, ginger and nutmeg (paan masala). The betel quid stains the teeth and oral mucosa red-brown to black [Figure - 6].[28] Betel chewing has been associated with premalignant conditions such as leucoplakia, oral submucous fibrosis and oral squamous cell carcinoma. It may also result in dental attrition. The retained betel quid along with trauma may result in an irregular brownish-red discoloration of mucosa known as 'Betel chewers mucosa'. Betel chewers mucosa tends to desquamate or peel off but is not considered premalignant.[29]
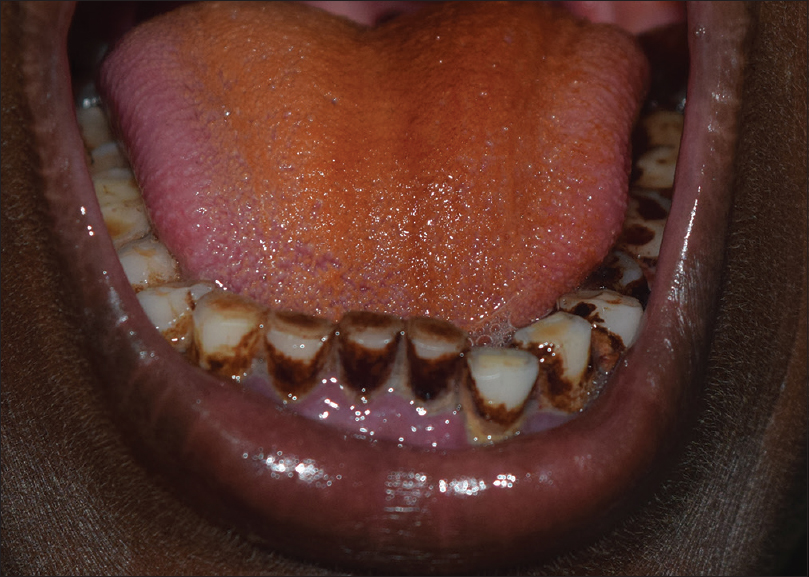 |
| Figure 6: Betel stains on the tongue and teeth |
Other plant sources of stains on teeth include tea (brown), coffee (brown), tobacco (dark-brown or black) and Khat or Catha edulis leaves (yellowish-brown). The teeth are traditionally stained black with a mixture of herbs, iron and burned wood in some Southeast Asian countries.[30]
Botanical Colorants in the Textile Industry
Plants have been used to dye textiles from antiquity. Indigo has its origins in ancient India. It is the most important and one of the oldest natural textile dyes and is hence known as 'king of natural dyes'. It continues to be universally popular due to the demand for naturally dyed blue denim. Plant sources of textile dyes with examples are listed in [Table - 2]. Some natural dyes require mordants (tannins, vegetable oils or metals such as aluminium, iron, tin, copper, chromium and zinc) to make the textile colorfast to light and washing.[31],[32],[33]
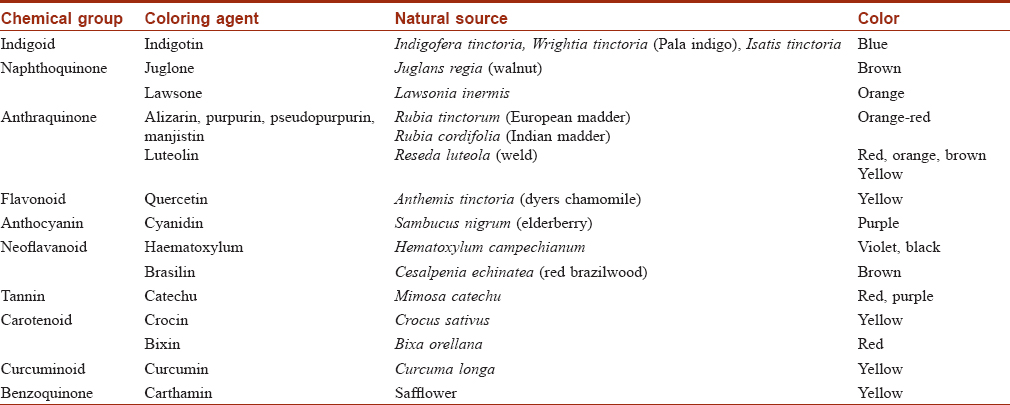
Dermatitis from the use of natural dyes is unusual and thus natural fibres dyed with natural dyes are recommended in textile dye hypersensitivity.[34] Natural dyes are renewable, bio-degradable and produce soft soothing colours. However, the production of natural dyes is a costly, tedious process with limitations in the range of colors that can be produced, reproducibility and color fastness. Toxicological evaluation for safety to humanity and environment is needed even for natural dyes. Some of the metallic mordants (eg., chromium, tin, copper) used to link natural dyes to fibres have adverse health or environmental effects.[31] Lawsone has potential for allergic contact dermatitis.[34]
Marking Nut Dermatitis
The Indian marking nut (Semecarpus anacardium) or washerman's nut or Dhobi's nut causes 'Dhobi-mark dermatitis' or marking nut dermatitis. The Indian marking nut tree belongs to the Toxicodendron genus and the family Anacardiaceae. The nut is black, shiny and smooth [Figure - 7] and yields a dark brown-black irritant juice or oil (bhilawan oil). Indian washerman (Dhobi) mix the juice with alum or lime water to leave an indelible mark on laundry. This had resulted in many cases of 'Dhobi-mark dermatitis' in British soldiers posted in India. The oleoresin urushiol, can produce both irritant and allergic contact dermatitis with severe pruritus, erythema, papules, urticaria, vesicles, bullae and erythema multiforme like lesions. Rarely renal toxicity may occur from systemic absorption.[20],[35]
 |
| Figure 7: The marking nut produces a dark brown juice |
Botanical Colourants in Food Additives
Food is said to be first eaten with the eye. Turmeric, bright red chillies and saffron are commonly used to increase the visual appeal of food in Indian cuisine (personal observation). Safety concerns over artificial additives have led to an increased demand for natural colorants in food. Common botanical food additives in food include turmeric (curcumin), carotenoids (beta-carotene, lycopene and canthaxanthin), beetroot (betalain), grape skin extract (enocianina), saffron (crocin) and annatto (bixin). Saffron has been implicated in anaphylaxis, and annatto in both urticaria and anaphylaxis. However, allergic reactions to botanical additives are extremely rare despite their widespread use.[36],[37]
Ingested Botanical Colorants
Several plant colourants are important in maintaining health and preventing diseases. Carotenoids, flavonoids (flavones, flavonols, anthocyanins, catechins and proanthocyanidin) and curcumin are widely studied for their anti-oxidant, anticancer and antiaging properties.[14],[38],[39] Carotenoids are natural organic compounds and include alpha- and beta-carotene, lycopene, beta-cryptoxanthin, lutein and zeaxanthin.[40] High carotene content is found in fruits such as mangoes, carrots, oranges, pineapple, papaya and some green leafy vegetables.[41] Carotenoids contribute to skin color and provide photoprotection. Oxycarotenoids such as lutein, are deposited in the macula lutea of the eye, and are protective against cataract and macular degeneration.[39] Carotenoids and flavonoids are often used as antioxidants in supplements to promote youthful skin. However, the efficacy and safety of supplements on the reactive oxygen species/antioxidant balance is uncertain and thus, direct consumption of fruits and vegetables is recommended.[42] Riboflavin (vitamin B2) is a yellow essential water soluble vitamin, synthesised by plants and microorganisms, which is crucial for several cellular functions.[43]
Carotenoderma
Carotenoderma or aurantiasis is the yellow-orange discoloration of the skin that most commonly occurs due to excess dietary intake of carotenoids.[44] Clinical manifestations of carotenoderma are seen when serum levels of beta-carotene are higher than 250 g/dl.[39] Yellow-orange pigment is deposited mainly in the stratum corneum and is prominent in areas with thick stratum corneum or increased sweating such as nasolabial folds, knees, palms and soles. Generalized pigmentation may occur. Sparing of the conjunctiva and enhanced pigmentation in artificial light distinguish carotenoderma from jaundice.[40] Intercellular autofluorescence resembling pemphigus vulgaris may be seen on direct immunofluorescence. Other causes of carotenoderma include diabetes mellitus, hypothyroidism, anorexia nervosa, nephrotic syndrome, liver diseases and familial carotonemia. The pathophysiology involves either hyperlipidemia or a failure of conversion of carotene to vitamin A or a combination of both.[41],[44] Differential diagnosis of carotenoderma includes jaundice, lycopenemia, riboflavinemia and xanthoderma secondary to drugs (sorafenib, sunitinib, quinacrine, dipyramidamole, saffron, canthaxanthin, fluorescein, acriflavine, picric acid, phenol and turmeric).[14],[40],[45],[46]
Dietary carotenoderma is a benign self-limiting condition. A low carotene diet produces resolution in weeks or months.[41]
Lycopenemia
Lycopenemia can occur due to high oral intake of lycopene. Lycopene is an inert carotenoid that is not converted to vitamin A. Tomatoes, beets, bittersweet berries, rose hips and chilli beans are rich sources of lycopene. Patients usually develop lycopenemia after ingestion of large quantities of tomatoes or tomato juice. Similar to carotenoderma, the conjunctiva is spared and the pigmentation can involve the palms and soles. However, the skin is more deeper orange than yellow and the colour can be distinguished with spectrophotometry. A combination of lycopenemia and carotenemia producing 'orange people' may occur after ingestion of yellow vegetables and tomatoes.[41],[47],[48] Lycopenemia can cause discolouration of the soft palate. A characteristic feature of lycopenemia is the deposition of lycopene in the liver, where it forms fatty cysts and alters the liver parenchyma. The pigmentation resolves after dietary restriction of lycopene.[38],[39]
Canthaxanthin
Canthaxanthin (β-carotene-4,4'-dione) is a natural carotenoid that was first identified in the mushroom, Cantherellus cinnabarinus. It is found naturally in some plants, bacteria, algae, crustacean, fish and birds, and can be synthesized. It has an orange to red color in oil or fat and is an approved food and drug color additive in the United states, Canada and Europe.[49]
Oral ingestion of canthaxanthin pills can impart a 'golden orange' or an orange-brown hue to the skin. It has been used to produce a 'healthy' complexion or simulate a tan (marketed as 'tanning pills'.[50] However, the stools become brick -red in colour with orange-brown discoloration of palms and soles. Canthaxanthin has been used as a photoprotective agent in photodermatosis (erythropoetic protoporphyria) and as a systemic non-melanin colouring agent (reduces contrast between vitiliginous and normal skin) in the treatment of vitiligo.[51],[52] Adverse effects include crystalline golden deposits in the retina, seizures, hepatitis, urticaria and aplastic anaemia.[40],[50]
Botanical Colorants in Histopathology
The earliest dyes used to colour tissue included indigo, madder and saffron.[53] Hematoxylin is one of the most popular natural histochemical stains. It remains unequalled by synthetic dyes. It is derived from the heartwood of the logwood tree, Hematotoxylum campechianum or 'blood-wood' tree. It was a valuable textile dye in Central America and Mexico. Hematoxylin is a colourless compound that oxidises to the reddish-brown dye 'hematein' on exposure to air. Mordants, such as alum, are used on 'hematein' to yield the permanent blue or black stain. In addition to routine haematoxylin and eosin staining, haematoxylin can be used to stain a wide range of tissue structures with the use of different oxidants, mordants and differentiating agents. Examples include Verhoeff's elastic stain, Weigerts iron-hematoxylin and Mallory's phosphotungstic acid hematoxylin.[54],[55]
Alizarin red is a calcium stain used in the diagnosis of disorders such as pseudoxanthoma elasticum and calcinosis cutis. The original alizarin was obtained from madder dye, which is composed of several anthraquinones from the roots of the madder plant (Rubia tinctorum). Madder is an ancient textile dye, medicinal plant and food colorant. Similar to hematoxylin, natural alizarin requires mordants such as alum to make it tissue fast. It has now been replaced by synthetic alizarin.[56]
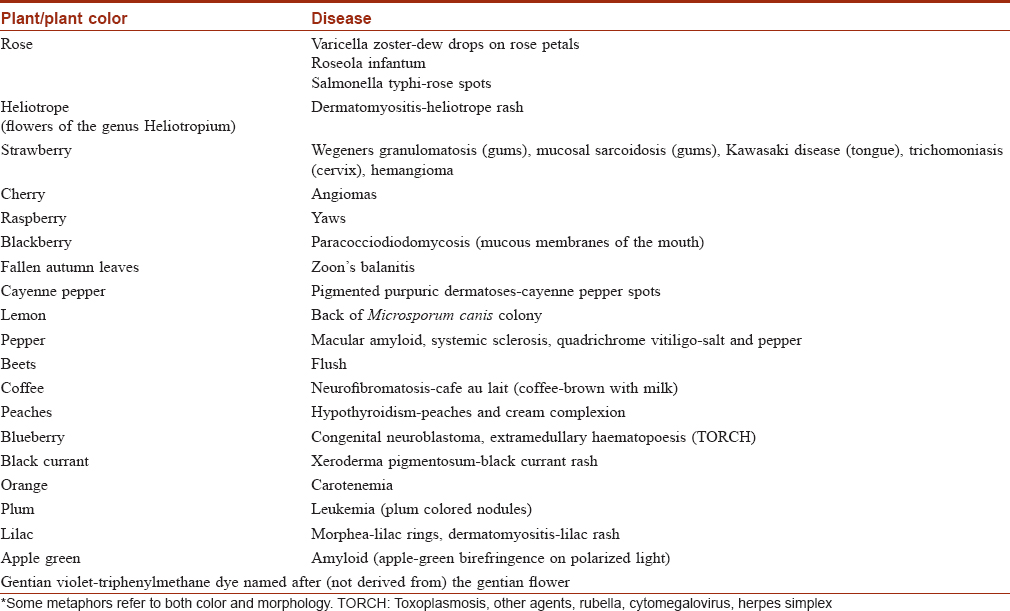
Botanical Colors as Dermatology Metaphors
Allusions to the botanical palette are abundant in our routine dermatology practice. Examples are given in [Table - 3].[57],[58],[59],[60],[61],[62],[63],[64],[65]
Conclusions
Although synthetic colorants have surpassed botanical colorants in several areas, some botanical colorants have stood the test of time and some are being re-explored for safer options. It is important to be aware of the uses of the spectacular botanical palette as it continues to inspire humanity to add a splash of color to our mundane appearance, our cuisine, and in the way we communicate in medicine.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Guichard S, Roulier V. Facial foundation. In: Draelos ZD, editor. Cosmetic Dermatology: Products and Procedures. 2nd ed.. Hoboken, New Jersey: Blackwell; 2010. p. 167-75.
[Google Scholar]
|
| 2. |
Melo MJ. History of natural dyes in the ancient mediterranean world. In: Bechtold T, Mussak R, editors. Handbook of Natural Colorants. Chichester: Wiley; 2009. p. 3-20.
[Google Scholar]
|
| 3. |
Davies KM, editor. Plant Pigments and their Manipulation. Oxford: Blackwell Publishing; 2004.
[Google Scholar]
|
| 4. |
Kazandjieva J, Grozdev I, Tsankov N. Temporary henna tattoos. Clin Dermatol 2007;25:383-7.
[Google Scholar]
|
| 5. |
de Groot AC. Side-effects of henna and semi-permanent 'black henna' tattoos: A full review. Contact Dermatitis 2013;69:1-25.
[Google Scholar]
|
| 6. |
Oumeish OY. The cultural and philosophical concepts of cosmetics in beauty and art through the medical history of mankind. Clin Dermatol 2001;19:375-86.
[Google Scholar]
|
| 7. |
Lewis DM, Rippon JA, editors. The Coloration of Wool and Other Keratin Fibres. Chichester: John Wiley & Sons; 2010.
[Google Scholar]
|
| 8. |
Redgrove HS, Foan GA, editors. Hair-dyes and Hair-dyeing Chemistry and Technique. London: Willian Heinemann; 1934.
[Google Scholar]
|
| 9. |
Aburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res 2003;17:987-1000.
[Google Scholar]
|
| 10. |
Calogiuri G, Di Leo E, Butani L, Pizzimenti S, Incorvaia C, Macchia L, et al. Hypersensitivity reactions due to black henna tattoos and their components: Are the clinical pictures related to the immune pathomechanism? Clin Mol Allergy 2017;15:8.
[Google Scholar]
|
| 11. |
Gupta D, Thappa DM. Dermatoses due to Indian cultural practices. Indian J Dermatol 2015;60:3-12.
[Google Scholar]
|
| 12. |
Bircher AJ, Sigg R, Scherer-Hofmeier K, Schlegel U, Hauri U. Allergic contact dermatitis caused by a new temporary blue–black tattoo dye–sensitization to genipin from jagua (Genipa americana L.) fruit extract. Contact Dermatitis 2017;77:374-8.
[Google Scholar]
|
| 13. |
Waton J, Brault F, Laveine E. A putative case of allergic contact dermatitis caused by a jagua tattoo. Contact Dermatitis 2017;76:296-7.
[Google Scholar]
|
| 14. |
Gopinath H, Karthikeyan K. Turmeric: A condiment, cosmetic and cure. Indian J Dermatol Venereol Leprol 2018;84:16-21.
[Google Scholar]
|
| 15. |
Vashi NA, Patzelt N, Wirya S, Maymone MBC, Kundu RV. Dermatoses caused by cultural practices: Cosmetic cultural practices. J Am Acad Dermatol 2018;79:19-30.
[Google Scholar]
|
| 16. |
Lee D, editor. Nature's Palette: The Science of Plant Color. Chicago: University of Chicago Press; 2007.
[Google Scholar]
|
| 17. |
Bicknell LM, Ladizinski B, Tintle SJ, Ramirez-Fort MK. Lips as red as blood: Areca nut lip staining. JAMA Dermatol 2013;149:1288
[Google Scholar]
|
| 18. |
Korkina L, De Luca C, Pastore S. Plant polyphenols and human skin: Friends or foes. Ann N Y Acad Sci 2012;1259:77-86.
[Google Scholar]
|
| 19. |
Neri I, Bianchi F, Giacomini F, Patrizi A. Acute irritant contact dermatitis due to Juglans regia. Contact Dermatitis 2006;55:62-3.
[Google Scholar]
|
| 20. |
Ghorpade A. Targetoid skin lesions with marking nut hair dye. Int J Dermatol 2015;54:1414-5.
[Google Scholar]
|
| 21. |
Bechtold T. Natural colorants in hair dyeing. In: Bechtold T, Mussak R, editors. Handbook of Natural Colorants. Chichester: Wiley; 2009. p. 339-50.
[Google Scholar]
|
| 22. |
Sonthalia S, Tiwary P. Colored dots on trichoscopy—beware of artifacts. J Am Acad Dermatol 2019;80 (6):e143-4.
[Google Scholar]
|
| 23. |
Ghosh SK, Bandyopadhyay D, Verma SB. Cultural practice and dermatology: The “Holi” dermatoses. Int J Dermatol 2012;51:1385-7.
[Google Scholar]
|
| 24. |
Science Behind Holi, the Festival of Colours. Available from: http://ncsm.gov.in/. [Last accessed on 2017 Jul 07].
[Google Scholar]
|
| 25. |
Gupta S, Selvan H, Markan A, Gupta V. Holi colors and chemical contact keratitis. Eye (Lond) 2018;32:1-3.
[Google Scholar]
|
| 26. |
Masavkar SS, Mauskar A, Patwardhan G, Bhat V, Manglani MV. Acquired methemoglobinemia – A sporadic holi disaster. Indian Pediatr 2017;54:473-5.
[Google Scholar]
|
| 27. |
Mack TM. The new pan-Asian paan problem. Lancet 2001;357:1638-9.
[Google Scholar]
|
| 28. |
Norton SA. Betel: Consumption and consequences. J Am Acad Dermatol 1998;38:81-8.
[Google Scholar]
|
| 29. |
Trivedy CR, Craig G, Warnakulasuriya S. The oral health consequences of chewing areca nut. Addict Biol 2002;7:115-25.
[Google Scholar]
|
| 30. |
Hattab FN, Qudeimat MA, al-Rimawi HS. Dental discoloration: An overview. J Esthet Dent 1999;11:291-310.
[Google Scholar]
|
| 31. |
Saxena S, Raja AS. Natural dyes: Sources, chemistry, application and sustainability issues. In: Muthu SS, editor. Roadmap to Sustainable Textiles and Clothing, Textile Science and Clothing Technology. Singapore: Springer Science + Business Media; 2014. p. 37-80.
[Google Scholar]
|
| 32. |
Mussak R, Bechtold T. Natural colorants in textile dyeing. In: Bechtold T, Mussak R, editors. Handbook of Natural Colorants. Chichester: Wiley; 2009. p. 315-38.
[Google Scholar]
|
| 33. |
Hill DJ. Is there a future for natural dyes?. Rev Prog Color Relat Top 1997;27:18-25.
[Google Scholar]
|
| 34. |
Coz L. Clothing. In: Johansen JD, Frosch PJ, Lepoitteven JP, editors. Contact Dermatitis. 5th ed.. Berlin: Springer; 2011. p. 793-817.
[Google Scholar]
|
| 35. |
Hafejee A, Coulson IH, Lakshiminarasimhan P, Leon CJ. Traditional tattoo treatment trauma. Clin Exp Dermatol 2008;33:536-7.
[Google Scholar]
|
| 36. |
Attokaran M, editor. Natural Food Flavors and Colorants. 2nd ed.. Chichester: Wiley-Blackwell; 2011.
[Google Scholar]
|
| 37. |
Lucas CD, Hallagan JB, Taylor SL. The role of natural color additives in food allergy. Adv Food Nutr Res 2001;43:195-216.
[Google Scholar]
|
| 38. |
La Placa M, Pazzaglia M, Tosti A. Lycopenaemia. J Eur Acad Dermatol Venereol 2000;14:311-2.
[Google Scholar]
|
| 39. |
Morganti P. The photoprotective activity of nutraceuticals. Clin Dermatol 2009;27:166-74.
[Google Scholar]
|
| 40. |
Haught JM, Patel S, English JC 3rd. Xanthoderma: A clinical review. J Am Acad Dermatol 2007;57:1051-8.
[Google Scholar]
|
| 41. |
Maharshak N, Shapiro J, Trau H. Carotenoderma – A review of the current literature. Int J Dermatol 2003;42:178-81.
[Google Scholar]
|
| 42. |
Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci 2017;85:152-61.
[Google Scholar]
|
| 43. |
Schwechheimer SK, Park EY, Revuelta JL, Becker J, Wittmann C. Biotechnology of riboflavin. Appl Microbiol Biotechnol 2016;100:2107-19.
[Google Scholar]
|
| 44. |
Gangakhedkar A, Somerville R, Jelleyman T. Carotenemia and hepatomegaly in an atopic child on an exclusion diet for a food allergy. Australas J Dermatol 2017;58:42-4.
[Google Scholar]
|
| 45. |
Ahluwalia J, Yan AC. Carotenoderma associated with a diet rich in red palm oil. Cutis 2018;101:E4-5.
[Google Scholar]
|
| 46. |
Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J 2007;100:328-30.
[Google Scholar]
|
| 47. |
Tung EE, Drage LA, Ghosh AK. Carotenoderma and hypercarotenemia: Markers for disordered eating habits. J Eur Acad Dermatol Venereol 2006;20:1147-8.
[Google Scholar]
|
| 48. |
Lascari AD. Carotenemia. A review. Clin Pediatr (Phila) 1981;20:25-9.
[Google Scholar]
|
| 49. |
Gupta AK, Haberman HF, Pawlowski D, Shulman G, Menon IA. Canthaxanthin. Int J Dermatol 1985;24:528-32.
[Google Scholar]
|
| 50. |
Lober CW. Canthaxanthin – The “tanning” pill. J Am Acad Dermatol 1985;13:660.
[Google Scholar]
|
| 51. |
Geoffrey BA, Felix MB. Canthaxanthin and the eye: A critical ocular toxicologic assessment. J Toxicol Cutan Ocul Toxicol 1991;10:115-55.
[Google Scholar]
|
| 52. |
Goldstein E, Haberman HF, Menon IA, Pawlowski D. Non-psoralen treatment of vitiligo. Part I. Cosmetics, systemic coloring agents, and corticosteroids. Int J Dermatol 1992;31:229-36.
[Google Scholar]
|
| 53. |
Titford M. Progress in the development of microscopical techniques for diagnostic pathology. J Histotechnol 2009;32:9-19.
[Google Scholar]
|
| 54. |
Titford M. The long history of hematoxylin. Biotech Histochem 2005;80:73-8.
[Google Scholar]
|
| 55. |
Norton SA. The useful plants of dermatology. II. Haematoxylum and hematoxylin. J Am Acad Dermatol 1996;34:149-51.
[Google Scholar]
|
| 56. |
Norton SA. Useful plants of dermatology. IV. Alizarin red and madder. J Am Acad Dermatol 1998;39:484-5.
[Google Scholar]
|
| 57. |
Sebaratnam DF. Apple of the dermatologist's eye. JAMA Dermatol 2014;150:1280.
[Google Scholar]
|
| 58. |
Jindal N, Jindal P, Kumar J, Gupta S, Jain VK. Fruit and food eponyms in dermatology. Indian J Dermatol 2015;60:213.
[Google Scholar]
|
| 59. |
Milam EC, Mu EW, Orlow SJ. Culinary metaphors in dermatology: Eating our words. JAMA Dermatol 2015;151:912.
[Google Scholar]
|
| 60. |
Sebaratnam DF, Lowe PM. Crazy in Love. JAMA Dermatol 2016;152:230.
[Google Scholar]
|
| 61. |
Vender R, Vender R. Gourmet clinical pearls. J Cutan Med Surg 2011;15:63-4.
[Google Scholar]
|
| 62. |
Mansouri B, Housewright CD. Dermatology's balanced diet. JAMA Dermatol 2016;152:1268.
[Google Scholar]
|
| 63. |
Madke B, Chougule BD, Kar S, Khopkar U. Appearances in clinical dermatology. Indian J Dermatol Venereol Leprol 2014;80:432-47.
[Google Scholar]
|
| 64. |
Mertens JS, Seyger MM, Thurlings RM, Radstake TR, de Jong EM. Morphea and eosinophilic fasciitis: An update. Am J Clin Dermatol 2017;18:491-512.
[Google Scholar]
|
| 65. |
Cooksey CJ. Quirks of dye nomenclature. 7. Gentian violet and other violets. Biotech Histochem 2017;92:134-40.
[Google Scholar]
|
Fulltext Views
11,386
PDF downloads
4,622





