Translate this page into:
Generalized asymptomatic erythematous nodules and plaques
China Xiu Fang Chen and Yi Zhan contributed equally to this article, they are both first authors
Corresponding author: Prof. Gui Ying Zhang, Department of Dermatology, The Second Xiangya Hospital of Central South University, Changsha 410011, China. lindazgy@csu.edu.cn
-
Received: ,
Accepted: ,
How to cite this article: Chen XF, Zhan Y, Li YP, Zhang J, Zhou Y, Zhang GY. Generalized asymptomatic erythematous nodules and plaques. Indian J Dermatol Venereol Leprol 2022;88:119-22.
A previously healthy 49-year-old male was referred to our department with the complaint of asymptomatic reddish swellings of 1 year duration. The lesions first developed on the extensor aspect of his right leg which gradually increased in size and thereafter more lesions appeared on the face and trunk. He had no fever or weight loss. There was no lymph node enlargement or sternal tenderness. Physical examination revealed multiple, firm, erythematous plaques and nodules on the face, trunk and right lower limb [Figure 1a-c]. There are not any abnormal cells detected in peripheral blood, BM smear and BM biopsy. Bone marrow cell morphology examination revealed medullary proliferative activity. Whole body positron emission tomography and computed tomography (PET-CT) scans revealed multiple enlarged lymph nodes in armpit and groin, which demonstrated the evidence of lymphoma involvement. A punch biopsy from on one of the nodules on the anterior chest was performed. Skin biopsies of the lesion on the anterior chest showed a diffuse and nodular infiltrate of medium-sized monomorphic tumor cells throughout the dermis and subcutaneous tissues [Figure 2a and b]. The results of immuophenotyping showed a high positive expression of CD123, CD4, CD56, CD68, CD43, LCA and TDT (60%+), while stains for MPO, Mum-1, EMA, CD20, CD79a, CD30, ALKp80, TIA-1, CK and EBER were negative [Figure 3a-c]. The positive range of Ki-67 was 90% [Figure 3d]. Clonal T-cell receptor gene rearrangements were negative.
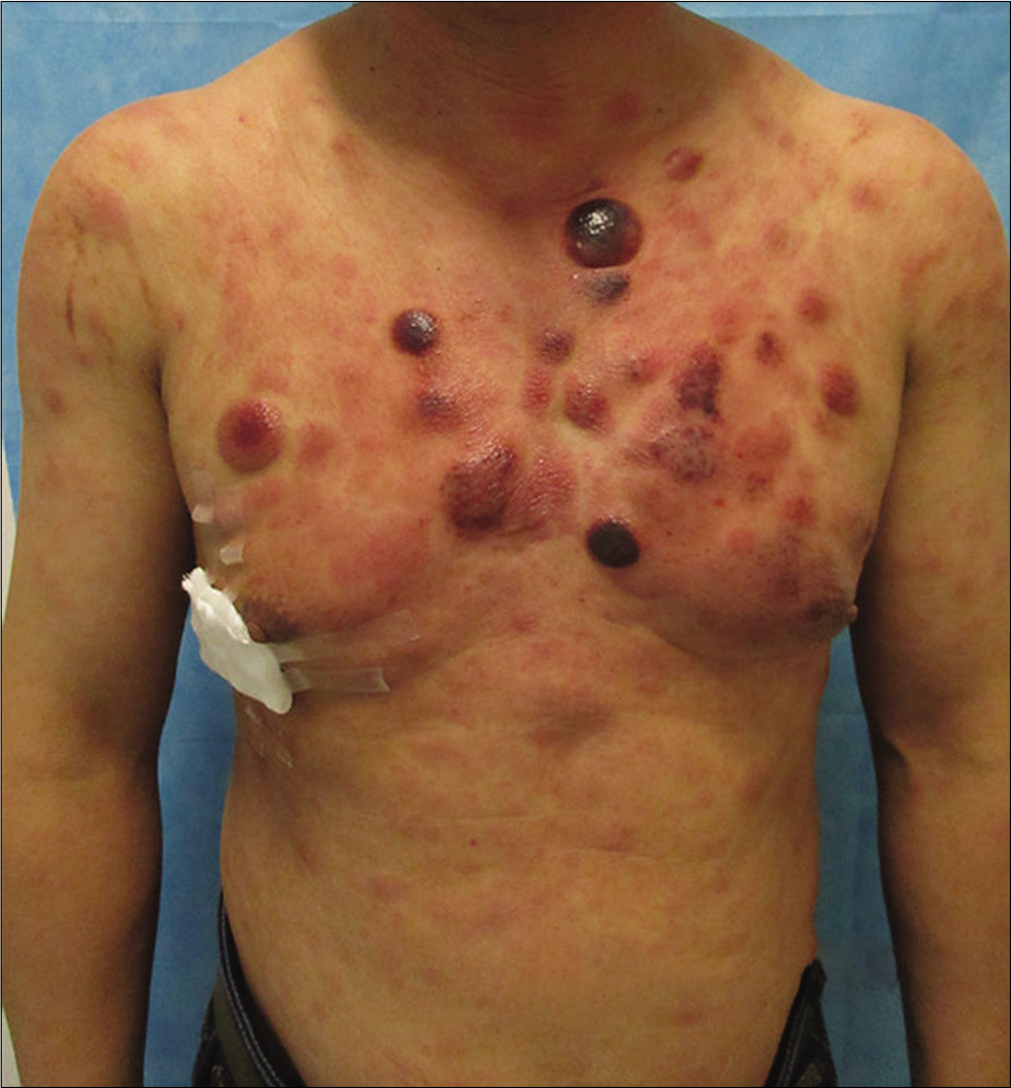
- Multiple erythematous plaques and nodules on the anterior aspect of trunk
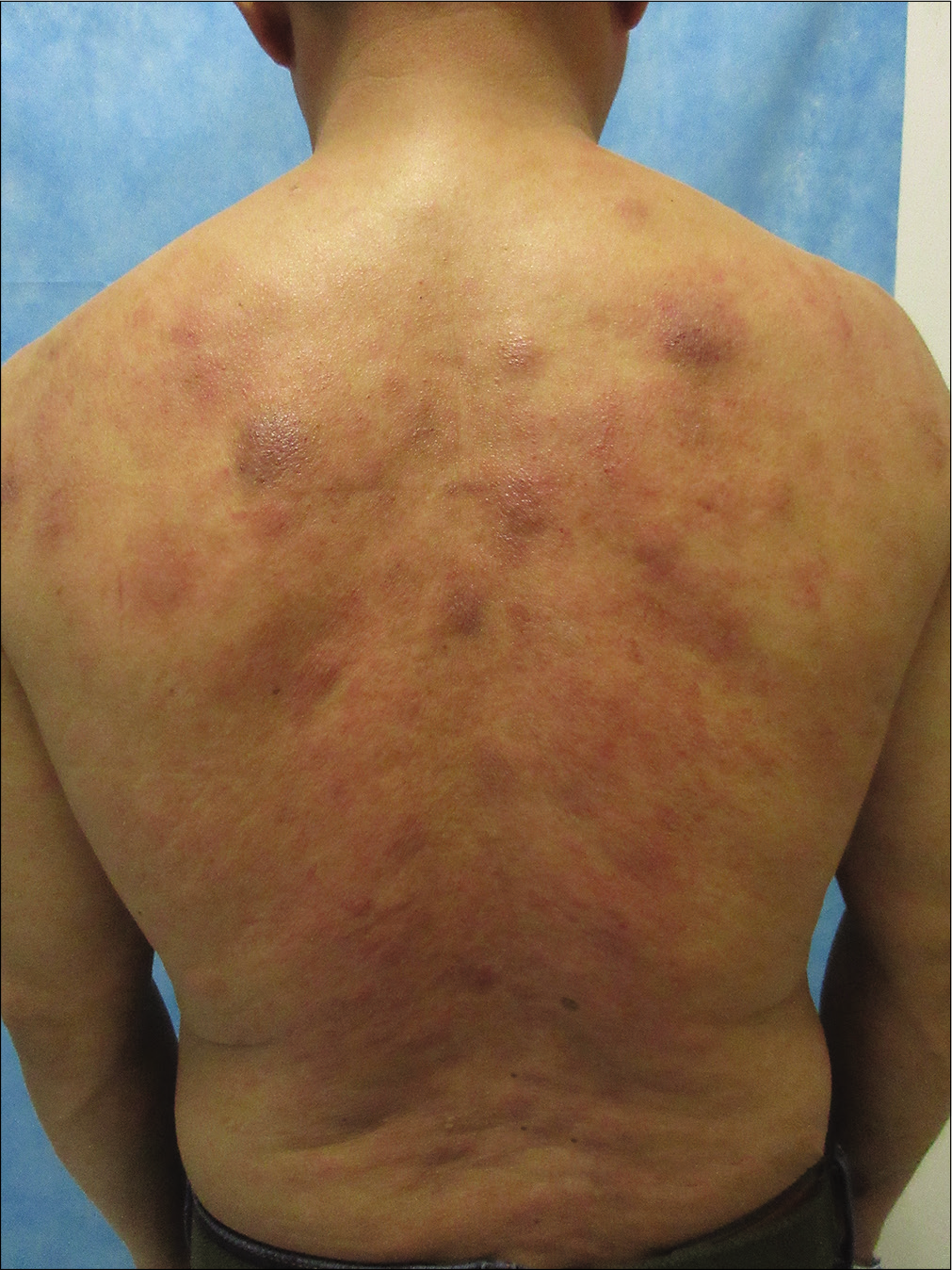
- Multiple erythematous plaques and nodules on the back
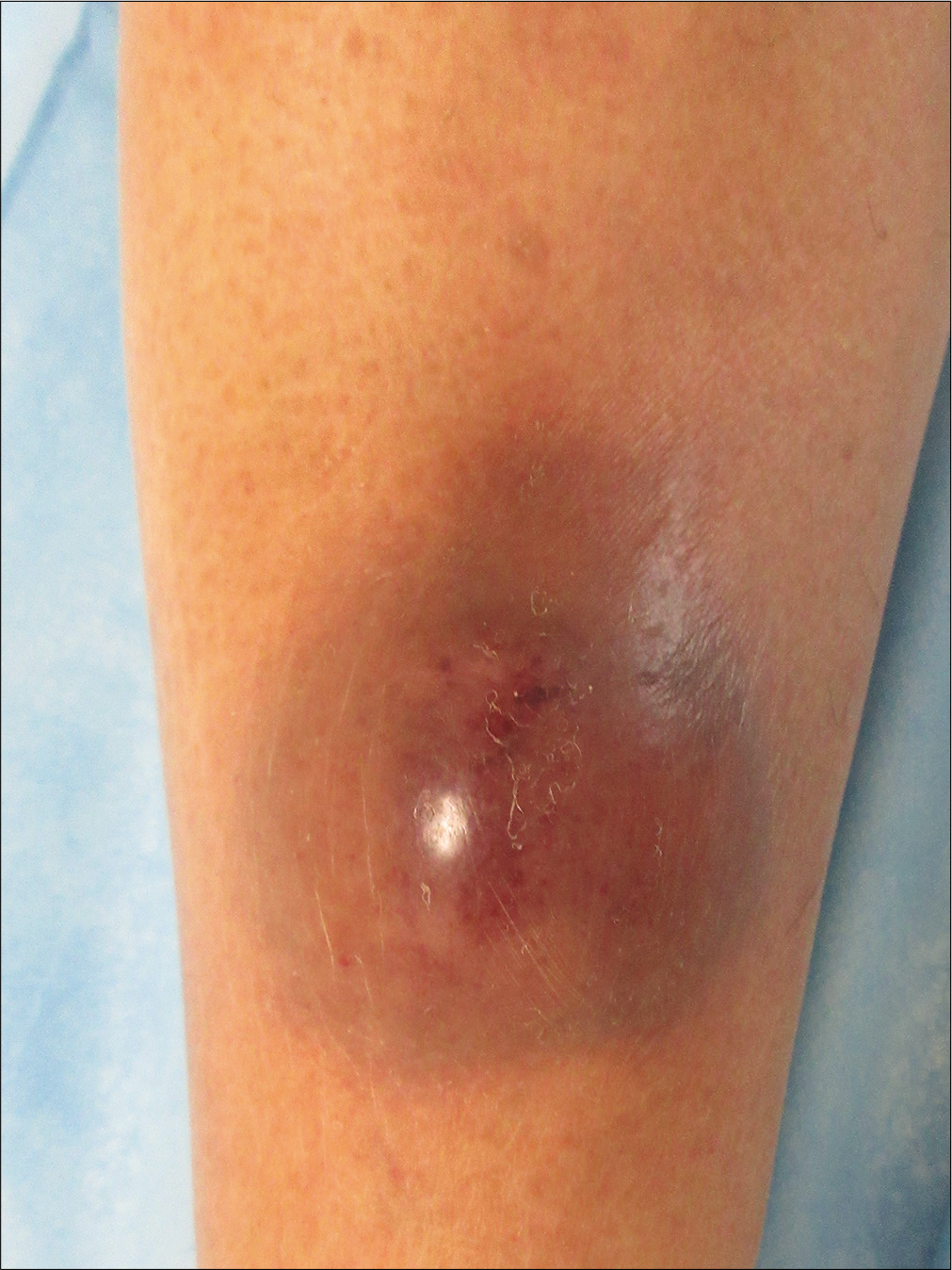
- An erythematous nodule on the extensor of the right leg
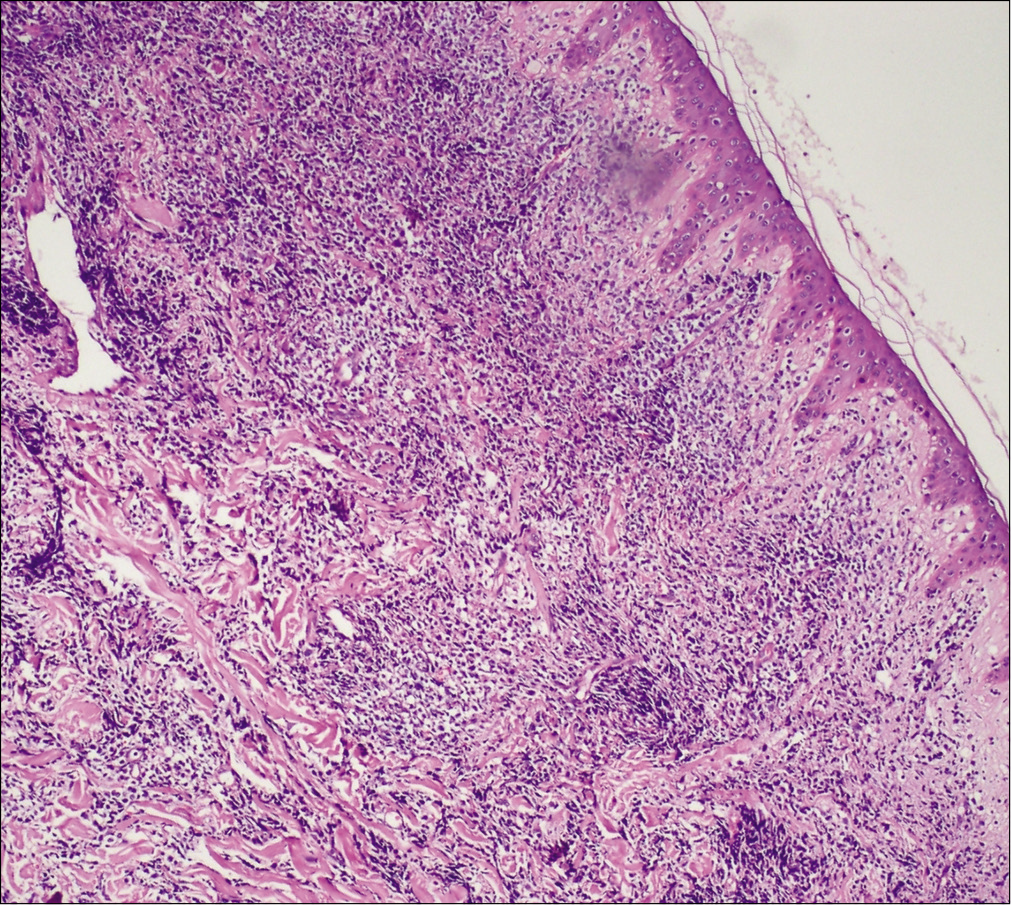
- A diffuse and nodular infiltrate of medium-sized monomorphic tumor cells throughout the dermis and subcutaneous tissues with a relatively normal epidermis (Hematoxylin and eosin, ×100)
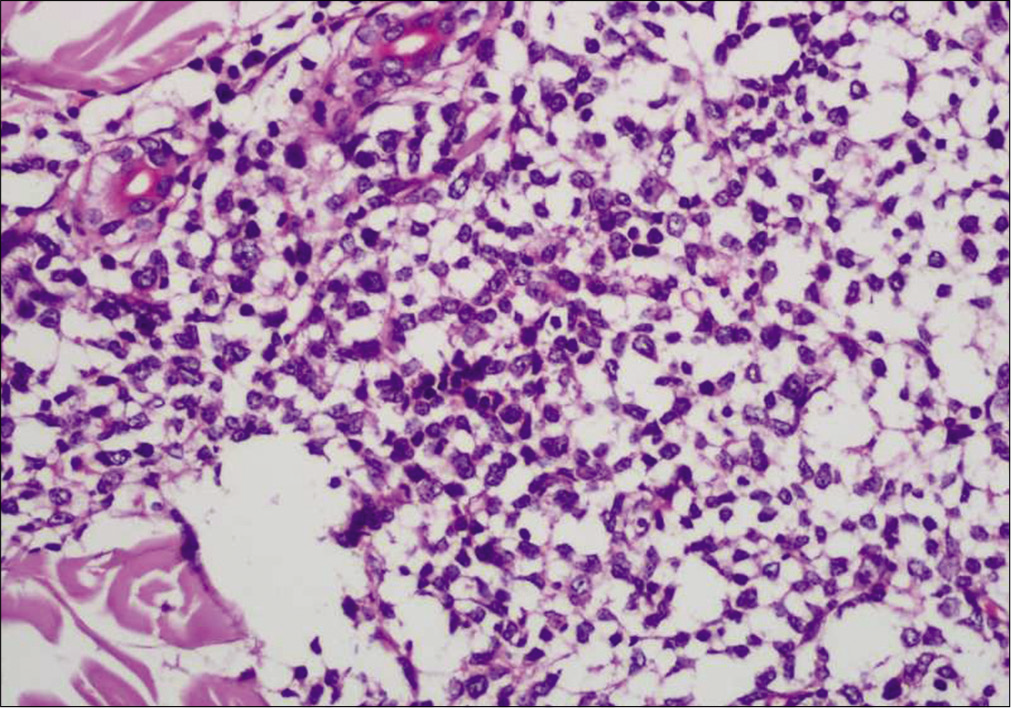
- Infiltrate of medium-sized monomorphic tumor cells (Hematoxylin and eosin, ×400)
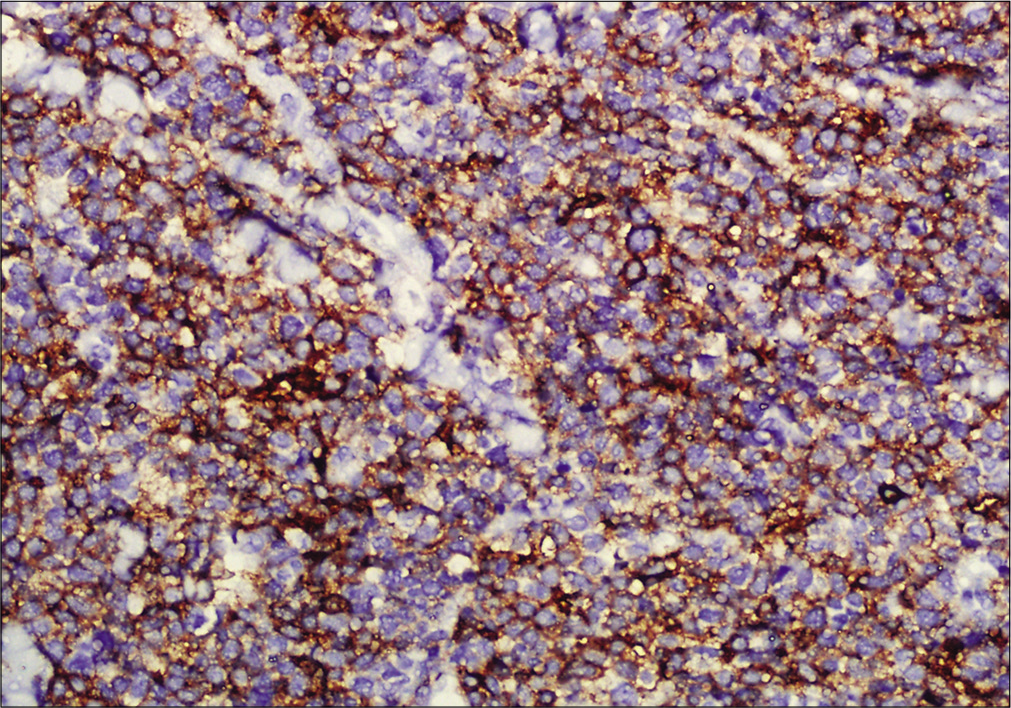
- Immunohistochemistry showing tumor cells positive for CD4 (×200)
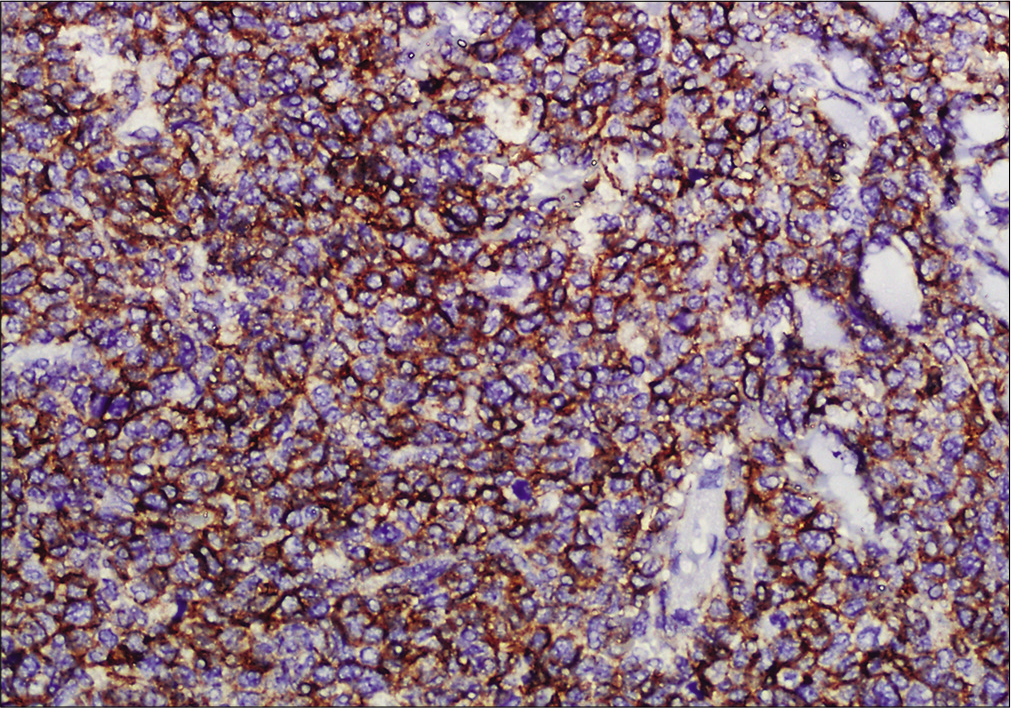
- Immunohistochemistry showing tumor cells positive for CD56 (×200)
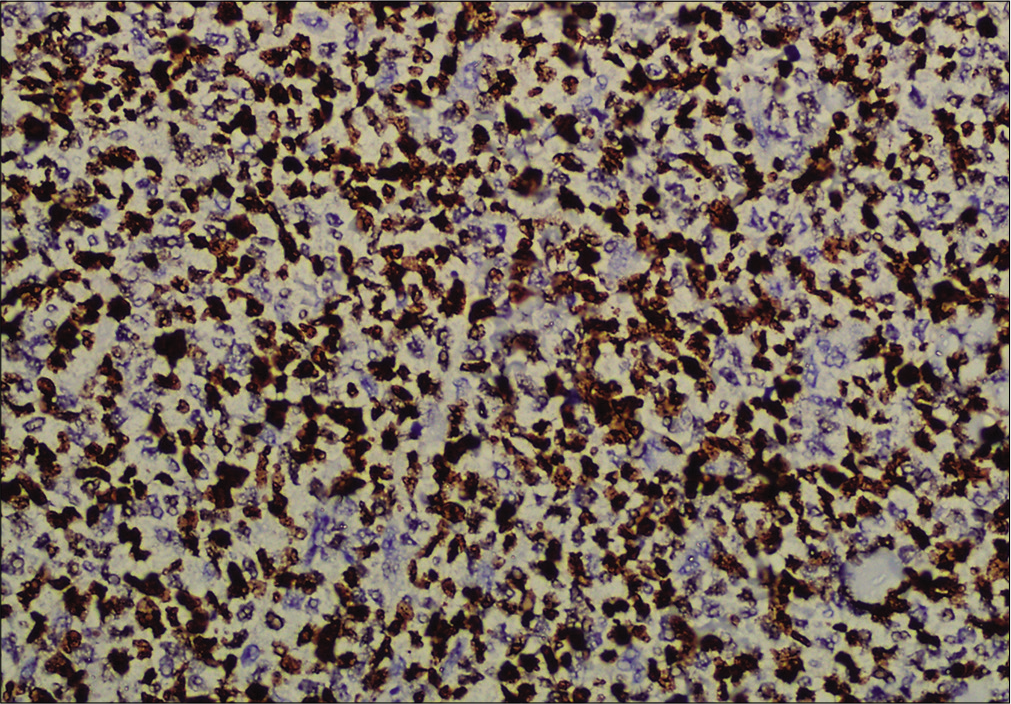
- Immunohistochemistry showing tumor cells positive for CD123 (×200)
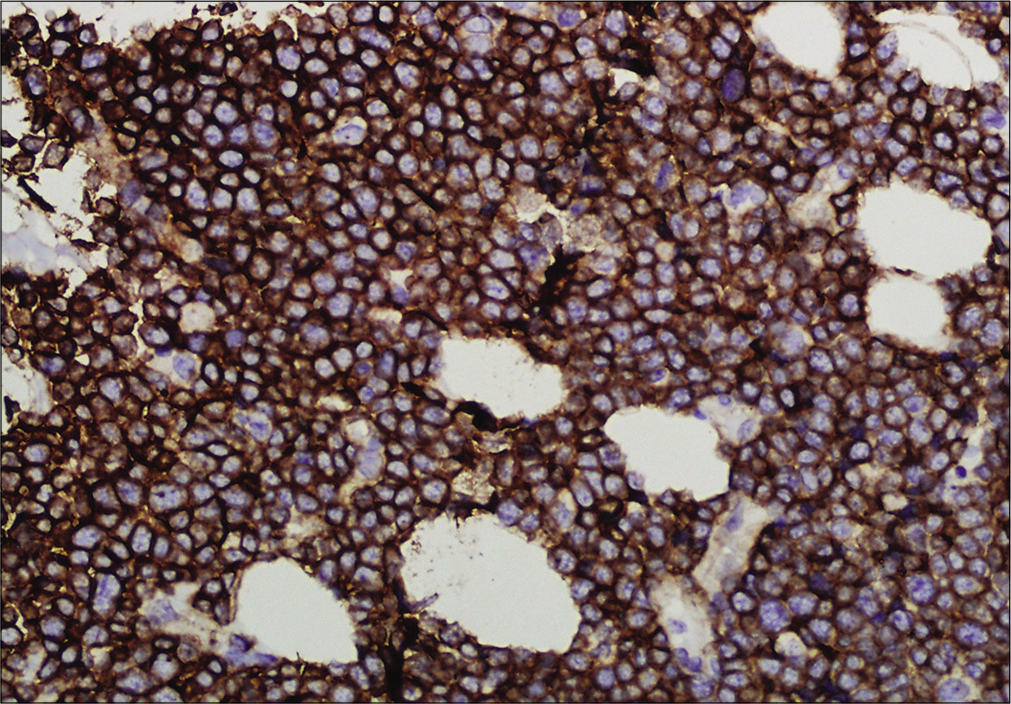
- Immunohistochemistry showing tumor cells positive for Ki-67 (×400)
Question
What is your diagnosis?
Answer
Primary cutaneous blastic plasmacytoid dendritic cell neoplasm.
Treatment and Follow-up
The patient was referred to the hematology department and attained partial remission after undergoing 2 rounds of chemotherapeutic regiem of EPOCH (etoposide, pirarubicin, vincristine, prednisone, cyclosphamide). However, he died of severe infection at 2 months of follow-up.
Discussion
Primary cutaneous blastic plasmacytoid dendritic cell neoplasm is a rare blastic plasmacytoid dendritic cells proliferative disorder that frequently involves the skin and bone marrow.1 Extracutaneous disease is common, including regional lymphadenopathy (55.8%), splenomegaly (44.2%), hepatomegaly (41.9%), central nervous system (9.3%) and pleural fluid (7.0%).2 This neoplasm commonly present on the skin as asymptomatic, nonspecific solitary or multiple nodules. Typical cutaneous lesions are large purplish plaques and nodules involving the head, neck and trunk. The expected pathological findings of primary cutaneous blastic plasmacytoid dendritic cell neoplasm are dense monomorphic infiltrate of medium-sized tumor cells with fine chromatin resembling lymphoblasts throughout the dermis with a well-defined grenz zone.3 Tumor cells express CD4, CD56 and CD123 without specific markers for myeloid, B, T and NK cells. Expression of TdT, a marker of the immature stage, significantly supported the less mature stages of primary cutaneous blastic plasmacytoid dendritic cell neoplasm.4 Atypical immunophenotype expression may be present, making diagnosis difficult. The clinical differential diagnosis includes myeloid sarcoma, acute myeloid leukemia, T-cell lymphoblastic leukemia/lymphoma, NK-cell lymphoma/ leukemia and chronic myelomonocytic leukemia with massive nodal/extranodal localization of plasmacytoid dendritic cells.5 The median survival time of patients was 15.2 months in a large case series.6 There is currently no consensus regarding optimal treatment for primary cutaneous blastic plasmacytoid dendritic cell neoplasm. Polychemotherapy, combination chemotherapy and radiotherapy, hematopoietic stem cell transplantation (HSCT) and target therapy are the mainstay treatments of primary cutaneous blastic plasmacytoid dendritic cell neoplasm. Pagano summarized 41 patients with induction therapy according to therapy for other hematopoietic malignancies such as acute leukemia and lymphoma.2 Seventeen patients achieved a complete remission, however relapse occurred in six of the patients. Combination chemotherapy and radiotherapy is another treatment option. Patients usually respond to initial chemotherapy but often relapse. Allogeneic or autologous bone marrow transplantation may be a better option. Roos-Weil et al. retrospectively reviewed 34 of primary cutaneous blastic plasmacytoid dendritic cell neoplasm patients who underwent allogeneic HSCT.7 16 patients (47%) were alive at 28 months (range 4-77) of follow up and 7 patients (32%) experienced relapse at a median of 8 months (range 2-27) after allo-SCT. Target therapy as an alternative way to treat primary cutaneous blastic plasmacytoid dendritic cell neoplasm is currently underway. Falcone et al. summarizes the different mechanisms of some target drugs, including hypomethylating agents, interleukin 3 receptor alpha inhibition, signaling pathway inhibition and monoclonal antibodies.8 Further clinical trials are still needed to verify therapeutic efficacy.
Acknowledgement
This research was supported by the National Natural Science Foundation of China (81500187, 81502710).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Blastic plasmacytoid dendritic cell neoplasm: A case report and literature review. Exp Ther Med. 2016;12:319--22.
- [CrossRef] [PubMed] [Google Scholar]
- Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: An Italian multicenter study. Haematologica. 2013;98:239--46.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological analysis of 46 cases with CD4+and/or CD56+immature haematolymphoid malignancy: reappraisal of blastic plasmacytoid dendritic cell and related neoplasms. Histopathology. 2017;71:972-84.
- [CrossRef] [PubMed] [Google Scholar]
- Blastic plasmacytoid dendritic cell neoplasm: From origin of the cell to targeted therapies. Biol Blood Marrow Transplant. 2016;22:1357-67.
- [CrossRef] [PubMed] [Google Scholar]
- Blastic plasmacytoid dendritic cell neoplasm: A clinicopathologic review. Arch Pathol Lab Med. 2014;138:564-9.
- [CrossRef] [PubMed] [Google Scholar]
- Blastic plasmacytoid dendritic cell neoplasms: Clinico-immunohistochemical correlations in a series of 91 patients. Am J Surg Pathol. 2014;38:673-80.
- [CrossRef] [PubMed] [Google Scholar]
- Stem cell transplantation can provide durable disease control in blastic plasmacytoid dendritic cell neoplasm: A retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2013;121:440-6.
- [CrossRef] [PubMed] [Google Scholar]
- A critical review of treatment modalities for blastic plasmacytoid dendritic cell neoplasm. Crit Rev Oncol Hematol. 2016;107:156-62.
- [CrossRef] [PubMed] [Google Scholar]





