Translate this page into:
Granuloma annulare on the palms: A clinicopathological study of seven cases
Correspondence Address:
Rameshwar Gutte
Department of Dermatology, Seth GS Medical College and KEM Hospital, Parel, Mumbai - 400 012, Maharashtra
India
| How to cite this article: Gutte R, Kothari D, Khopkar U. Granuloma annulare on the palms: A clinicopathological study of seven cases. Indian J Dermatol Venereol Leprol 2012;78:468-474 |
Abstract
Background : Granuloma annulare (GA), a common dermatological condition of unknown etiology, affecting all ages. Involvement of the palms appears rare, posing a diagnostic challenge. This study was conducted to document various clinical and histopathological features of GA on the palms. Aims: To study clinical and histopathological variations in granuloma annulare on palms. Methods : A total 7 patients from our outpatient department with lesions of GA, exclusively on the palms, were studied over a period of 6 months from March 2011 to August 2011. Total of 8 biopsies were studied. In each patient, diagnosis was made on clinico-pathological correlation. Various clinical and histopathological features were analyzed. Results: An average age of onset was 47 years, male: female ratio was 1.33: 1, and average disease duration was 9 months. Pain was the most common symptom. Clinically, the most common presentation was pseudovesicles. Histologically, both classic palisading and interstitial pattern were seen. Perineural granulomas, perieccrine granulomas, elastophagocytosis were seen additionally. In 5 out of 7 patients, diagnosis was missed clinically. Conclusion: GA on palms poses a diagnostic challenge due to variable presentations. Histopathology is of vital importance for correct diagnosis and treatment. GA should be considered in differential diagnosis of papular lesions on palms.Introduction
Granuloma annulare (GA) is a common, benign skin condition, described first over a century ago, but still remains an enigma with respect to etiology, associated systemic diseases and treatment. A number of clinical variants have been classified. Involvement of palms in GA is rare. [1] Palmar GA has varied morphological presentations and poses a diagnostic challenge. Skin biopsy is of vital importance to make the correct diagnosis and treatment in such cases. We studied 7 patients of GA on palms over a period of 5 months. The aim of the present study was to characterize various clinical presentations and histopathological variations in lesions of GA on palms.
Methods
A total of 7 patients from our outpatient department with lesions of GA exclusively on palms, with or without any other skin disease were enrolled over a period of 5 months. All the patients were subjected to detailed history, clinical examination and skin biopsy after an informed consent. In each patient, the diagnosis was made based on clinico-pathological correlation. Clinical variables, such as age, sex, duration of disease, symptomatology, type of lesions, distribution of lesions over palms, previous treatments taken if any and previous clinical diagnosis were studied. Variables considered in histopathological examination included epidermal changes, pattern of infiltrate, type of infiltrate, vascular changes, collagen and elastin tissue alterations, appendageal involvement and dermal mucin. Findings from all the patients were tabulated and analyzed to characterize various clinicopathological features of GA on palms.
Results
Clinical
We identified a total of 7 patients of GA with lesions exclusively on palms over a period of 5 months.
An average age of disease onset was 47 years. The youngest patient was 42 year old while the oldest was 74 year old. Males were more commonly affected than females with a male: female ratio 1.33:1.
An average duration of disease was 9 months with the shortest duration being 1 month while the longest being 3 years. 4 out of 7 lesions were recurrent with variable interval between episodes. No other associated skin disease was seen in any patient. 1 patient had history of insulin dependent diabetes since 10 years, and 1 patient had history of chronic low backache and was on aceclofenac, paracetamol and calcium supplement. Pain was the most common symptom, seen in 4 out of 7 patients, followed by itching in 2 out of 7 patients while 2 patients were asymptomatic.
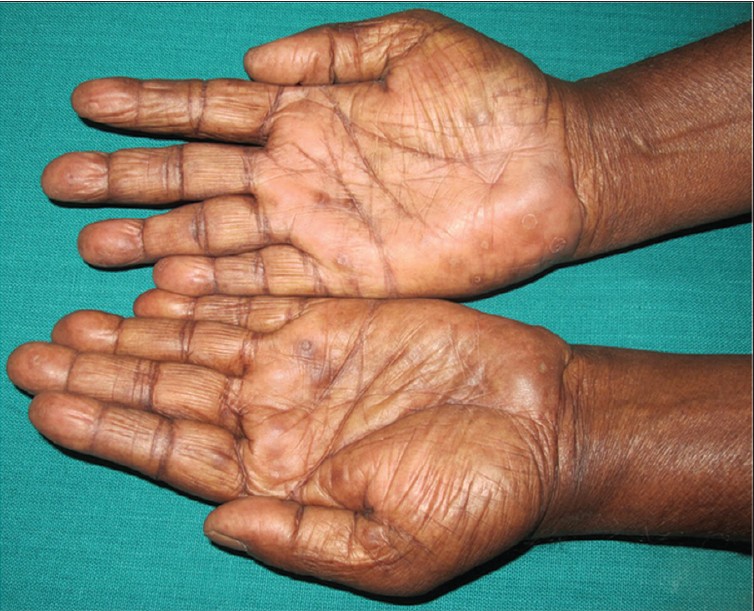
Nail involvement was seen in 1 patient. Blackish discoloration of nails with nail dystrophy and Beau′s lines were seen [Figure - 1]. Patient complained of repeated attacks of subungual pain and swelling of digit, which used to start simultaneously with lesions on palms and leading to nail dystrophy subsequently. Potassium hydroxide mount and culture from nail scraping were negative for fungal elements. Patient denied nail biopsy and occurrence of GA in nail matrix with subsequent nail dystrophy was speculated though not confirmed.
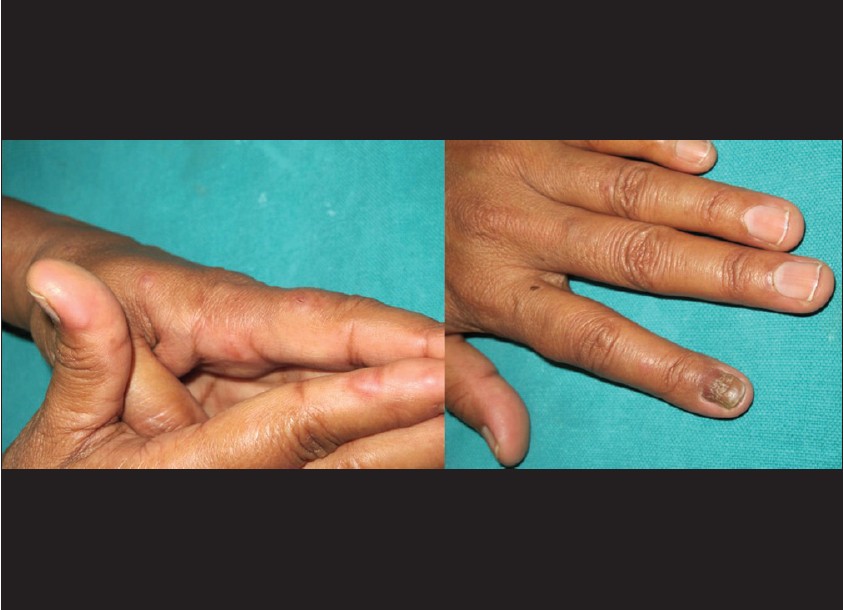 |
| Figure 1: Multiple pseudovesicular lesions on palmar aspect of fingers and dystrophy of index finger nail with multiple transverse ridges and discoloration is seen |
Common site affected on palms were finger tips followed by thenar and hypothenar eminence, lateral sides of fingers. In 2 patients, lesions were symmetrical while asymmetrical lesions were seen in remaining 5 out of 7 patients.
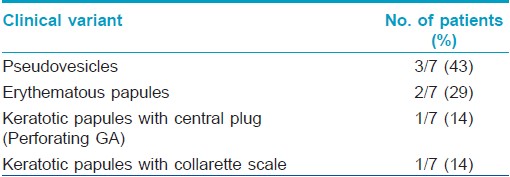
Morphologically pseudovesicles [Figure - 1] and [Figure - 2] erythematous papules [Figure - 3] followed by hyperkeratotic papules with central plug were the most common presentation [Figure - 4]. Keratotic papules with collarette scales were seen in 1 case, mimicking secondary syphilis [Figure - 5]. However, no serological evidence of syphilis was found. In a patient with keratotic papules with central plug, perforation of epidermis was seen histologically.
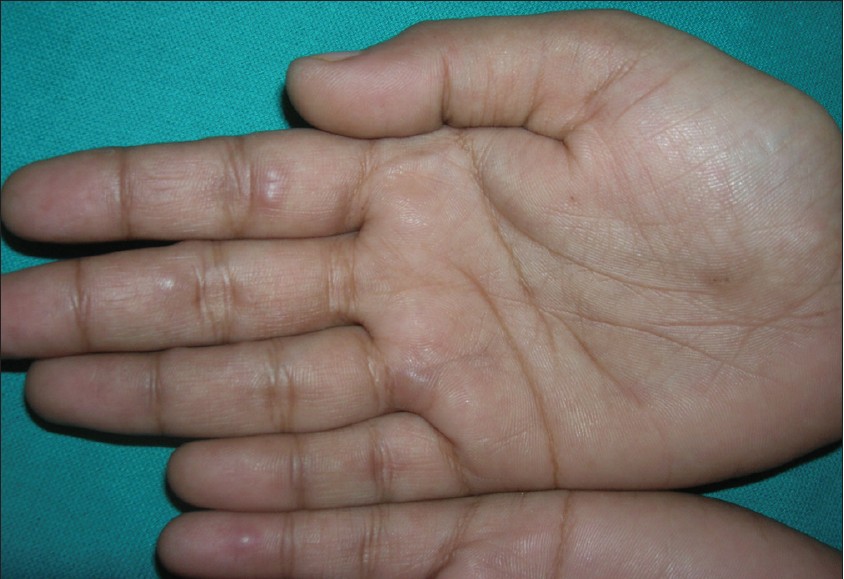 |
| Figure 2: Multiple pseudovesicular lesions, mainly on fingers are seen |
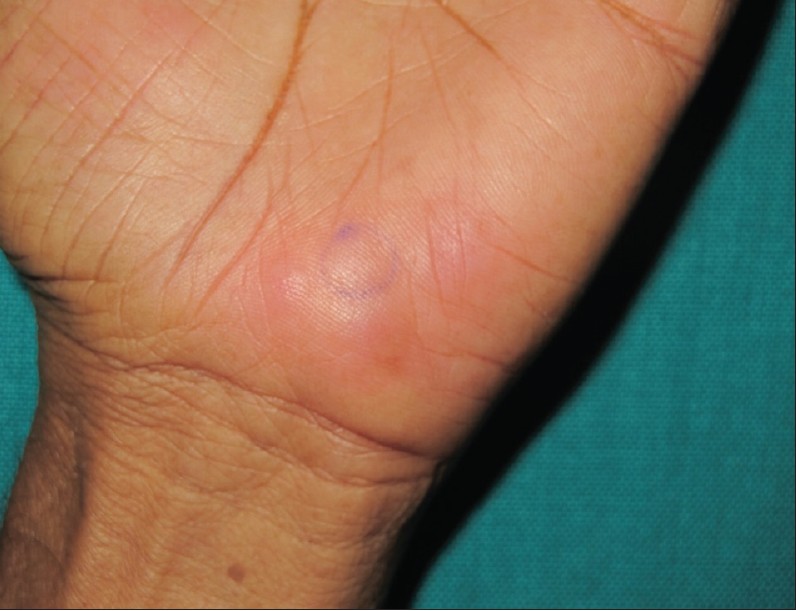 |
| Figure 3: Multiple erythematous papules over hypothenar aspect, clinically mimicking vasculitis or urticaria |
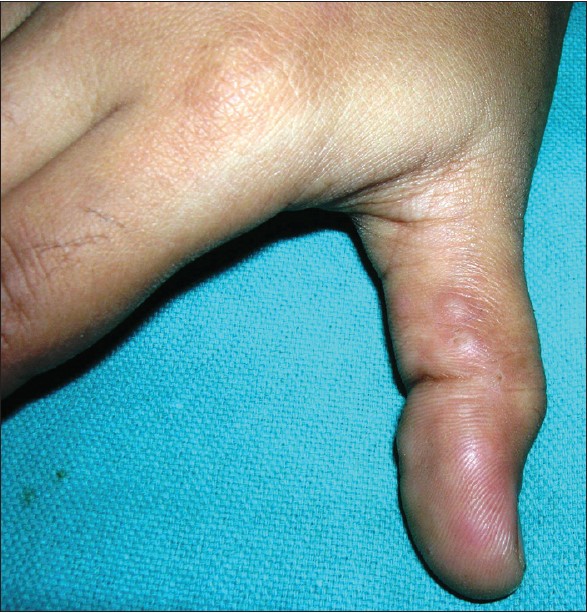 |
| Figure 4: Multiple keratotic papules over left thumb with central tiny plugs (Perforating GA) |
 |
| Figure 5: Multiple keratotic papules with collarette scales over bilateral palms |
In 5 out of 7 patients, diagnosis was missed clinically, and various other clinical diagnoses included vasculitis, erythema multiforme, pompholyx, Id eruption, palmar warts, drug reaction, urticaria and secondary syphilis in 1 case.
Various morphological variants seen and their percentage are shown in [Table - 1].
Histopathological
A total of 8 biopsies were obtained from palmar region of 7 patients. Almost all biopsies showed characteristic features of granuloma annulare with some additional features in 2 cases.
The patient in [Figure - 1] had multiple recurrences with dystrophy of nail. First biopsy showed classical features of GA with lympho-histiocytic palisading granuloma in upper dermis, surrounding abundant mucin. Repeat biopsy at third episode with painful pseudovesicles showed perineural and perieccrine granulomas in addition to classic features of GA [Figure - 6].
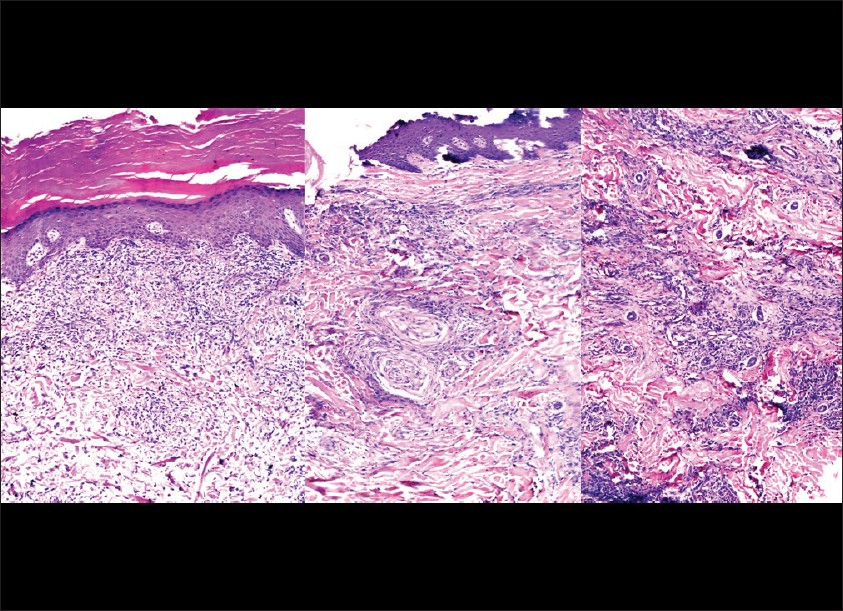 |
| Figure 6: Biopsy from a papulovesicular lesion showing classic palisading granuloma in superficial dermis. Repeat biopsy at 3rd episode from same patient revealed perineural and perieccrine granulomas. (H and E, ×100) |
Abundant solar elastosis with elastophagocytosis was seen in a patient, presenting with hyperkeratotic papules with collarette scales [Figure - 5] along with an interstitial pattern of GA. Presence of elastotic material within multinucleated giant cells was confirmed by Verhoff von-Gieson stain [Figure - 7].
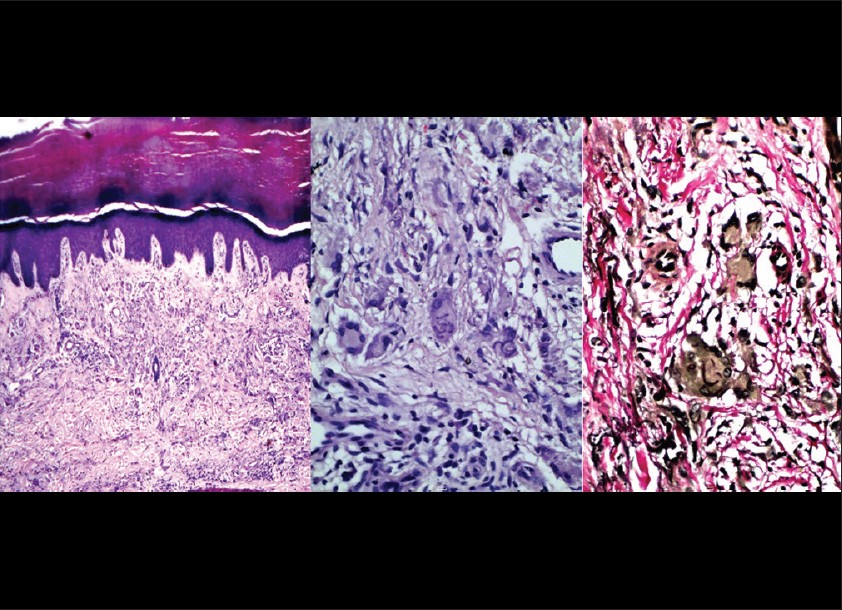 |
| Figure 7: Mixed pattern of granuloma annulare with abundant solar elastosis and elastophagocytosis is seen. (H and E, ×100). Elastic fibers in giant cell are stained with VVG stain (VVG, ×100) |
In 4 out of 7 patients, histopathology showed classical GA with palisading granuloma around mucin deposition and areas of collgenolysis. Presence of mucin was confirmed by alcian blue stain at pH 2.5 [Figure - 8].
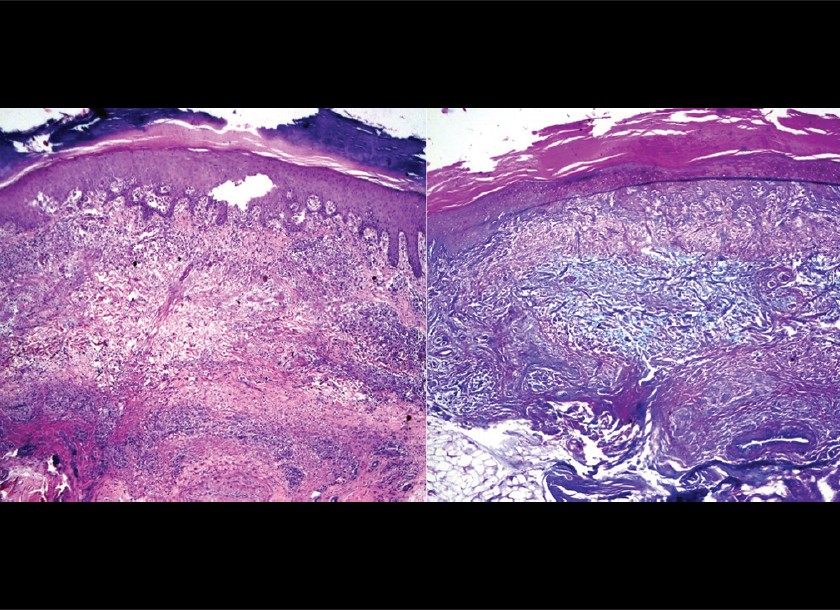 |
| Figure 8: A classic palisading granuloma around abundant mucin collection is seen. (H and E, 100X) Mucin deposition confirmed by alcian blue stain. (Alcian blue, ×100) |
A mixed pattern with both interstitial and classical palisading granulomas was seen in 1 patient. There was also thickening and degeneration of collagen bundles [Figure - 9]. No subcutaneous GA was observed in our study.
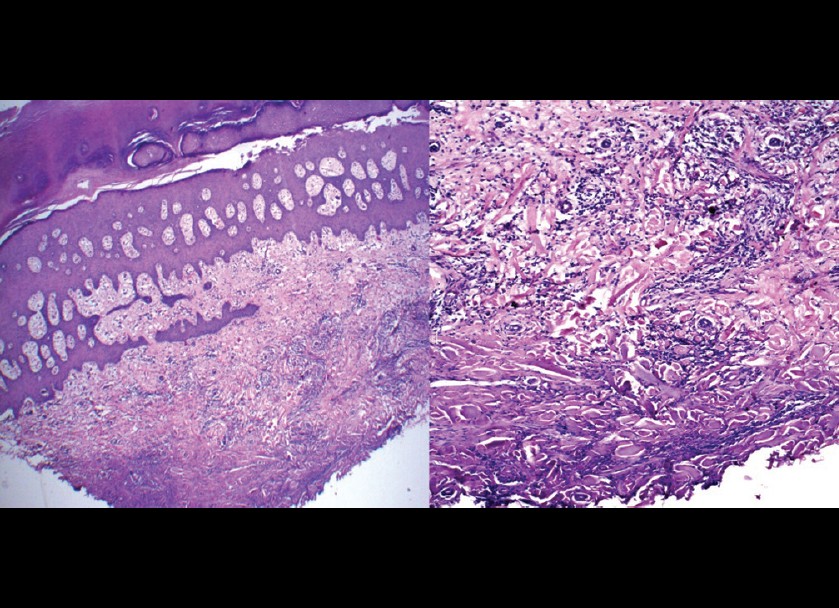 |
| Figure 9: Interstitial pattern of GA with poorly formed palisades is seen. Also, thickening and increased basophilia of collagen fibers is seen. (H and E, ×100) |
In patient, who presented with keratotic papules with central plug, perforation of epidermis was seen histologically. The perforating material was composed of degenerated collagen, mucin and granulomatous infiltrate [Figure - 10]. An interstitial pattern of GA with interstitial lymphohistiocytic infiltrate and mucin deposition, without formation of proper palisades, was seen in 2 out of 7 patients [Figure - 11]. No major vascular changes were observed in our study, except for endothelial swelling in 2 cases. Few eosinophils and plasma cells were seen in 2 cases only. Also, neutrophilic nuclear dust was seen in 2 cases.
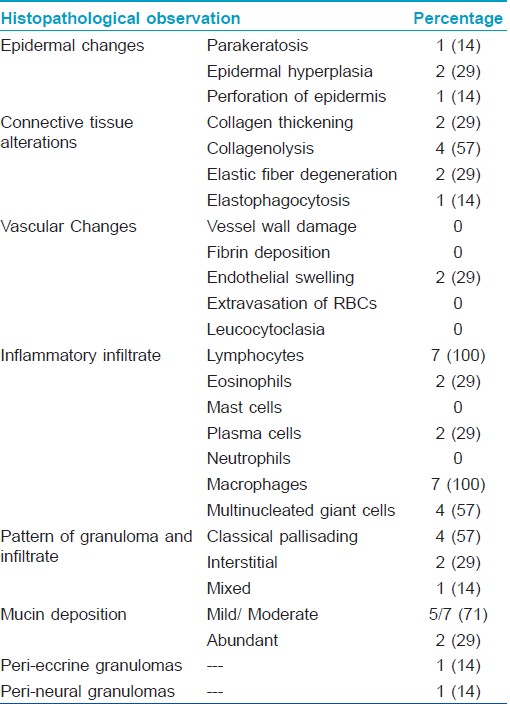
No atypia or abnormal mitoses were seen in our study. Mucin deposition was abundant in 2 cases while mild interstitial mucin deposition was seen in 2 cases. Moderate mucin, both interstitially and in the center of palisading granuloma was seen in 3 cases. Various histological parameters and their percentage are shown below in [Table - 2].
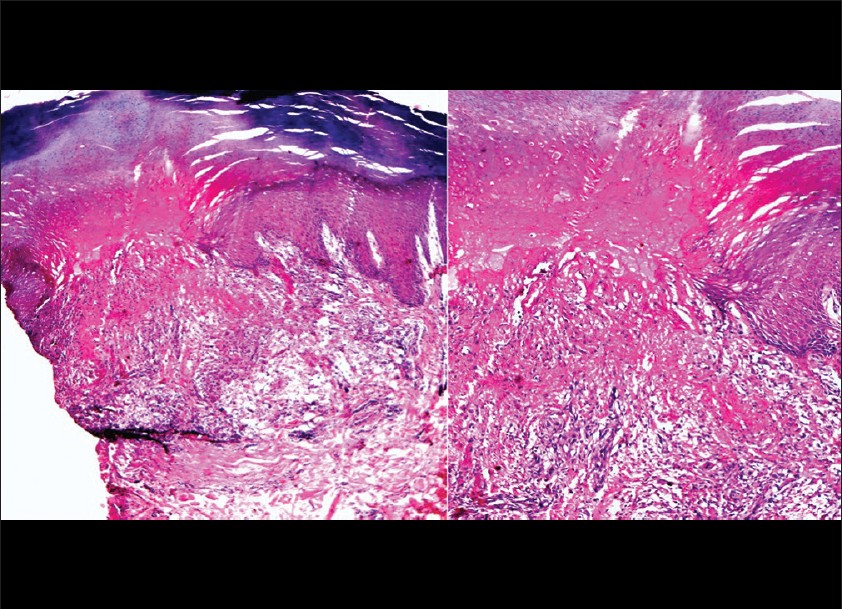 |
| Figure 10: A granulomatous infiltrate containing degenerated collagen and mucin and perforating acanthotic epidermis is seen. (H and E, ×100, ×200) |
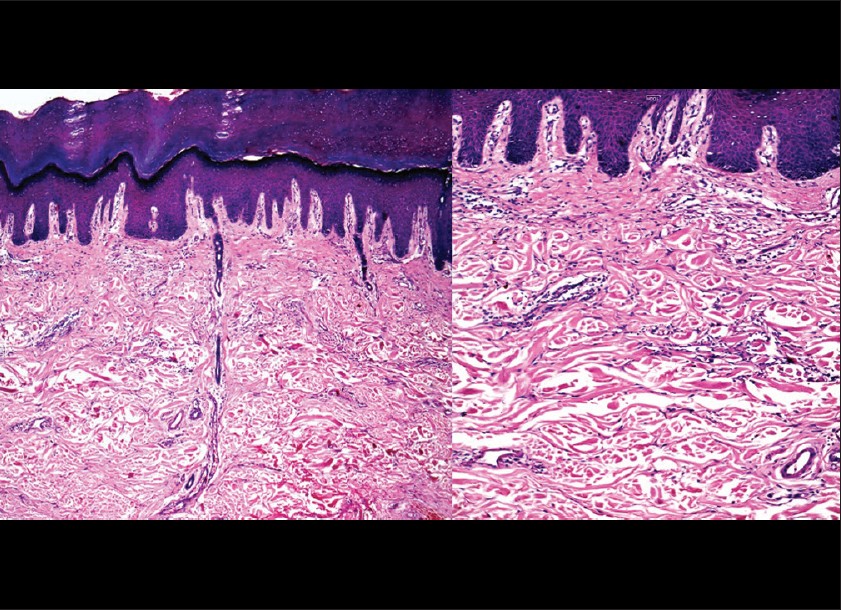 |
| Figure 11: Classical interstitial pattern of GA is seen with interstitial lymphohistiocytic infiltrate and stringy mucin deposition in dermis. (H and E, ×100, ×200) |
Discussion
Granuloma annulare (GA) is a benign, relatively common cutaneous disease that classically presents as localized clusters of small papules, coalescing to form annular plaques. It often occurs symmetrically, and lesions are typically asymptomatic. 2/3 rd of patients are under 30 years of age, and it is approximately twice more common in females than in males. Numerous clinical variants of GA have been described with overlapping features including generalized, papular, nodular, patch, perforating and subcutaneous GA.
It often favors acral sites such as the dorsum of the hands, elbows, and feet and involvement of the palms appears to be rare. [1]
Many clinicopathological studies have been done for generalized GA. Due to the rarity, no large sample study with clinicopathological correlation has been done for GA on the palms.
GA on the palms has variable clinical presentations, and diagnosis may be missed without histopathology. In our study, diagnosis was missed clinically in 5 out of 7 patients.
Various clinical presentations of palmar GA reported include painful acral papules, erythematous tender annular plaques, violaceous papules, indurated plaques, subcutaneous nodules, keratotic papules with central plug (Perforating GA) etc. [1],[2],[3] In our study, pseudovesicles was most common presentation followed by erythematous papules. Also, we found perforating GA in 1 case and keratotic papules with collarette scales in 1 case.
Various epidermal changes seen included, perforation of epidermis with transepidermal elimination of altered collagen in 1, parakeratosis in 1 and epidermal hyperplasia in 2 cases. Irregular epidermal acanthosis and epidermal atrophy has been reported to occur in GA. [2],[3]
Annular elastolytic giant cell granuloma (AEGCG), a rare granulomatous dermatosis of controversial origin, characterized by loss of elastic fibers and elastophagocytosis by multinucleated giant cells and lesions are usually located on sun-exposed areas.
The term AEGCG was proposed by Hanke et al., in 1979 and includes the lesions previously called actinic granuloma of O′Brien, atypical necrobiosis lipoidica and Miescher′s granuloma. Our observation of solar elastosis with elastophagocytosis gives credence to the speculation that AEGCG is not a separate entity, but granuloma annulare occurring on sun exposed areas. Moreover, elastophagocytosis is also seen in other conditions like temporal arteritis, sarcoidosis etc. and is not a specific feature. [2],[4],[5] Thus, the various confusing nominations like, AEGCG, actinic granuloma of O′Brien and granuloma disciform[is of face (Miescher′s granuloma) seems to be superfluous and should be avoided.
We found collagen tissue alteration like collagen thickening, increased basophilia, loss of normal fibrillary structure and collagenolysis was seen in 2 and 4 out of 7 cases. Günes et al ., found collagen degeneration of varying intensity in all 38 biopsies they studied and proposed that primary events in the pathogenesis of GA involve collagen as well as elastic fiber damage. [2],[4] In study by Stefanaki et al., also, necrobiosis with collagen degeneration was constant histological finding, irrespective of depth of infiltrate. [6] In a study by Yun et al., 28 out of 54 patients showed necrobiotic collagen with classic palisading granulomas while interstitial pattern without much collagen alteration was seen in 26 patients. [7]
We did not find significant vascular changes in our study. No vessel wall damage, fibrin thrombi or extravasation of RBCs was seen. Endothelial swelling was seen in 2 cases. Günes et al ., found, that endothelial swelling was observed in 18 (52.2%) specimens, and perivascular edema was observed in 27 (71%) specimens. Perivascular and predominantly lymphocytic inflammation was observed in 32 (84.2%) specimens. While neither fibrin thrombi nor leukocytoclasis in vessel walls was detected in any of the specimens, mild erythrocyte extravasation was observed in 2 (5.2%) and they argued that vascular changes are not related etiologically to GA. [2] Hong et al., found endothelial swelling in 59%, vasculitis and nuclear fragmentation in 36% cases each. [8]
Silverman and Rabinowitz specifically studied eosinophils in cellular infiltrate of GA. They found eosinophils in 18/45 (40%) cases of GA among which, over half the cases were of deep GA while 1/3 rd of the cases were of superficial GA. [9]
2 basic patterns of histiocytic infiltration are seen in GA, classic palisading and interstitial although both may be seen in same specimen comprising of a mixed pattern. We found classic palisading pattern in 4 cases, interstitial pattern in 2 cases and mixed pattern in 1 case out of 7. Hong et al., found classic palisading pattern in 46% cases and interstitial pattern in 50% of cases in their study. [8] Yun et al., found palisading pattern in 52% and interstitial pattern in 48% cases while Kim KH found palisading pattern in 27% and interstitial pattern in 46% cases. [10]
Mucin deposition was seen in all biopsies in our study. It was mild to moderate in 71% and abundant in 29% cases. Yun et al., found mucin deposition in 51 cases (94%). Günes et al., found dermal mucin deposition in 32 (84.2%) specimens. Mucin deposition was observed in 19/22 (86.4%) specimens with interstitial patterns, 8/10 (80%) specimens with palisading patterns, 4/3 (75%) specimens with mixed pattern and 2/2 (100%) specimens with sarcoidal granulomatous patterns. [2] No sarcoidal granuloma was seen in our study.
Occurrence of mitotic figures in GA is rare but well-known feature, and 1 to 7 mitotic figures per 10 high-power fields have been reported. Recognition of this is important to avoid over diagnosis of malignant conditions, especially epitheloid sarcoma. [11] No mitotic figures were seen in any specimen of our study.
One of the interesting finding seen in our study was presence of peri-eecrine and perineural granulomas in a biopsy from a patient who complained of severe pain. These findings were not present initially, but were seen in a repeat biopsy done during an acute episode of the same patient who showed classical palisading granuloma initially.
A case of a granulomatous drug reaction with GA-like lesions of the palms as well as the legs in a 64-year-old woman, occurring 13 days after starting amlodipine and clearing within 3 months of drug cessation has been reported. [12] 1 of our patients was taking aceclofenac-paracetamol combination along with calcium supplements with antacids before getting lesions on palms. However, she had been taking these since 1 year prior to the sudden onset of her skin lesions, making these a less likely cause of her eruption. She also lacked the extra palmar, intertriginous annular plaques that clinically typify interstitial granulomatous drug reactions.
In conclusion, GA on palms has got varied clinical presentation, and diagnosis is often missed clinically. Histopathology is important in diagnosis of such cases to avoid unnecessary treatments. Histopathological features seen in our study were comparable to studies on generalized GA. Solar elastosis, elastophagocytosis, peri-neural and peri-eccrine granulomas were additional features found in our study.
| 1. |
Stewart LR, George S, Hamacher KL, Hsu S. Granuloma annulare of the palms. Dermatol Online J 2011;17:7.
[Google Scholar]
|
| 2. |
Güneº P, Göktay F, Mansur AT, Köker F, Erfan G. Collagen-elastic tissue changes and vascular involvement in granuloma annulare: A review of 35 cases. J Cutan Pathol 2009;36: 838-44.
[Google Scholar]
|
| 3. |
Brey NV, Malone J, Callen J. Acute-onset, painful acral granuloma annulare: A report of 4 cases and a discussion of the clinical and histologic spectrum of the disease. Arch Dermatol 2006;142:49-54.
[Google Scholar]
|
| 4. |
Dabski K, Winkelmann RK. Generalized granuloma annulare: Histopathology and immunology: Systematic review of 100 cases and comparison with localized granuloma annulare. J Am Acad Dermatol 1989;20:28-39.
[Google Scholar]
|
| 5. |
Ventura F, Vilarinho C, Duarte M, Pardal F, Brito C. Two cases of annular elastolytic giant cell granuloma: Different response to the treatment. Dermatol Online J 2010;16:11.
[Google Scholar]
|
| 6. |
Stefanaki K, Tsivitanidou-Kakourou T, Stefanaki C, Valari M, Argyrakos T, Konstantinidou CV, et al. Histological and immunohistochemical study of granuloma annulare and subcutaneous granuloma annulare in children. J Cutan Pathol 2007;34:392-6.
[Google Scholar]
|
| 7. |
Yun JH, Lee JY, Kim MK, Seo YJ, Kim MH, Cho KH. Clinical and pathological features of generalized granuloma annulare with their correlation: A retrospective multicenter study in Korea. Ann Dermatol 2009;21:113-9.
[Google Scholar]
|
| 8. |
Hong SJ, Kim DJ, Son SJ, Kang H. The clinicopathological study of granuloma annulare. Korean J Dermatol 1999;37:1029-37.
[Google Scholar]
|
| 9. |
Silverman RA, Rabinowitz AD. Eosinophils in the cellular infiltrate of granuloma annulare. J Cutan Pathol 1985;12:13-7.
[Google Scholar]
|
| 10. |
Kim KH, Shur KB, Lee JH, Park JK. Clinicopathological study of granuloma Annulare. Korean J Dermatol 2001;39:861-4.
[Google Scholar]
|
| 11. |
Trotter MJ, Crawford RI, O'Connell JX, Tron VA. Mitotic granuloma annulare: A clinicopathologic study of 20 cases. J Cutan Pathol 1996;23:537-45.
[Google Scholar]
|
| 12. |
Magro CM, Crowson AN, Schapiro BL. The interstitial granulomatous drug reaction: A distinctive clinical and pathological entity. J Cutan Pathol 1998;25:72-8.
[Google Scholar]
|
Fulltext Views
8,574
PDF downloads
2,754





