Translate this page into:
Haim Munk syndrome: Report of two siblings of Northern India treated with acitretin
Correspondence Address:
Raviprakash Sasankoti Mohan
c/o Dr. R. P. Singh, Dhanwantri Nursing Home, Sarai Khalsa, Behind Head Post Office, Moradabad (U.P.) 244 001
India
| How to cite this article: Mohan RS, Verma S. Haim Munk syndrome: Report of two siblings of Northern India treated with acitretin. Indian J Dermatol Venereol Leprol 2011;77:252 |
Abstract
Haim Munk Syndrome (HMS) is the allelic mutation of exon 6 codon in cathepsin C gene. Here, we present two cases of same family with HMS having all the cardinal features of HMS which includes palmo plantar keratoderma and periodontitis along with arachnodactyly, acroosteolysis, onychogryphosis, and marked osteopenia on hand wrist radiographs. Both the siblings were treated with cotrimoxazole, acetretin and topical keratolytics and followed up over a period of one year, showed remarkable improvement in palmo plantar keratoderma and periodontitis.Introduction
Haim-Munk syndrome (HMS) is also known as Cochin Jewish disorder or congenital keratosis palmoplantaris. Features include callous patches of skin on the palms and soles, long pointed fingers, and degeneration of the tissues that surround and support the teeth. HMS is attributed to a point mutation of the cathepsin C gene. Boys and girls are equally affected, with no racial predominance. Consanguinity has been observed in one-third of the cases described with HMS. [1]
We present two children of a Muslim family with HMS whose severe palmoplantar keratoderma and periodontal disease responded dramatically to treatment with cotrimoxazole, acetretin and topical keratolytics.
Case Report
An eleven- year old boy and his six year old sister reported to the out-patient department complaining of loose teeth. Patients also complained of peeling of the skin of the hands and feet.
Family history revealed consanguinity as the parents were first cousins.
Extra oral examination of the elder boy revealed abnormality of the skin in the form of keratoderma on the palmo plantar aspects of hands, feet, lateral aspect of the legs, knees and elbows; nail abnormalities in the form of onychogryphosis; finger abnormalities in the form of pointed terminal phalanges and feet abnormalities in the form of pes planus [Figure - 1] and [Figure - 2] while the younger girl also revealed palmo plantar keratoderma on the hands and feet but was less severe when compared to her brother [Figure - 3].
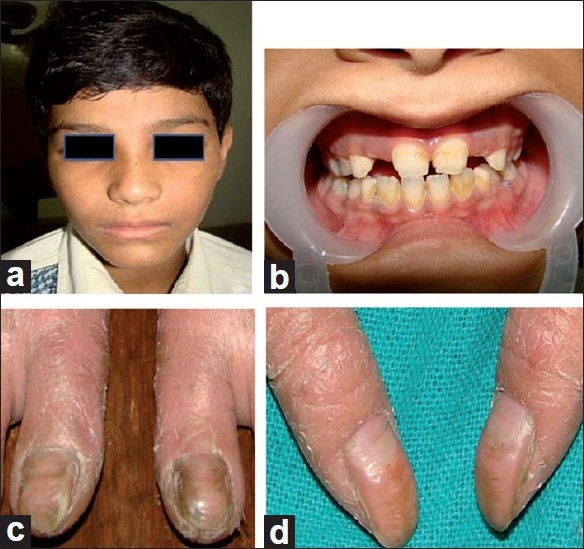 |
| Figure 1: Photographs of the elder boy. (a) Extra oral profile, (b) Periodontal status, (c) Onychogryphosis of toes, (d) Onychogryphosis of thumbs |
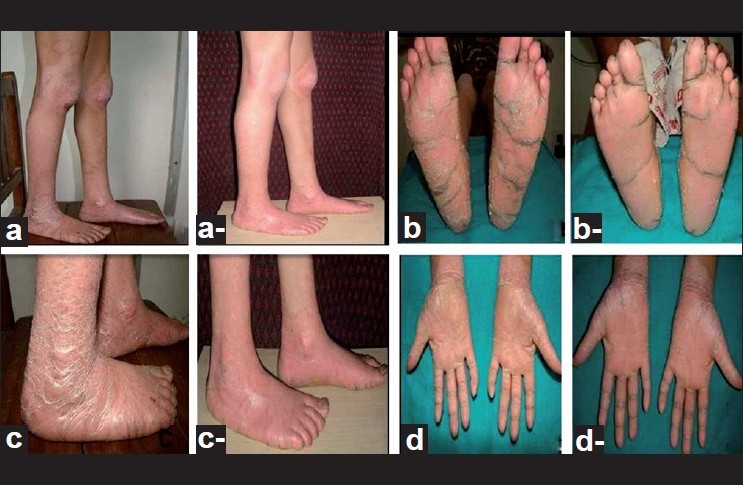 |
| Figure 2: Photographs of elder boy. (a,a-) Lateral view of pre treatment and post treatment photograph of keratoderma on the legs. (a, a-) Pre treatment and post treatment photograph of keratoderma on both soles. (c,c-) Lateral view of pre treatment and post treatment photograph of keratoderma on both the feet. (d,d-) Pre treatment and post treatment photograph of keratoderma on the palmar aspect of both hands |
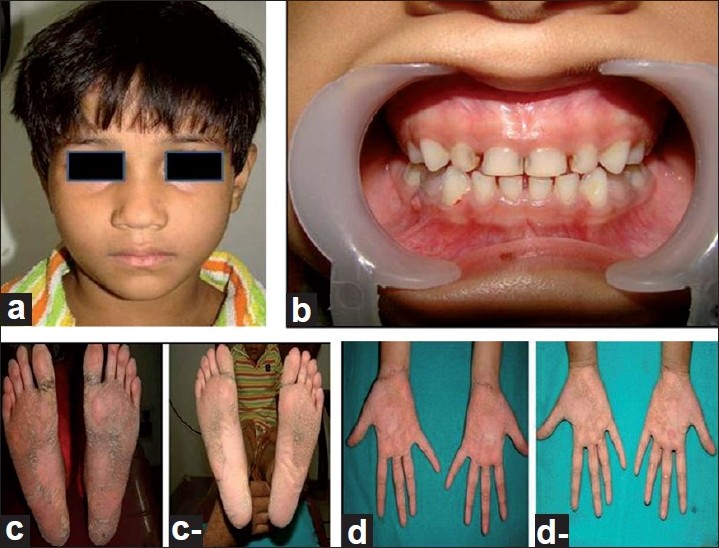 |
| Figure 3: Photographs of younger girl. (a) Extra oral profile. (b) Periodontal status. (c,c-) Pre treatment and post treatment photograph of keratoderma on the soles. (d,d-) Pre treatment and post treatment photograph of keratoderma on the palmar aspect of both hands |
On intraoral examination, both the siblings had mixed dentition with, deep periodontal pockets along with mobility affecting all the teeth [[Figure - 1]b] and [Figure - 3]b].
Orthopantomograms revealed generalized destruction of alveolar bone which was less severe in the younger girl [[Figure - 4]a]; skull projections did not reveal any calcific areas within the skull and; hand wrist radiographs revealed marked osteopenia in both the siblings and acroosteolysis of the right index finger and little finger in the elder boy. [[Figure - 4]b, c]
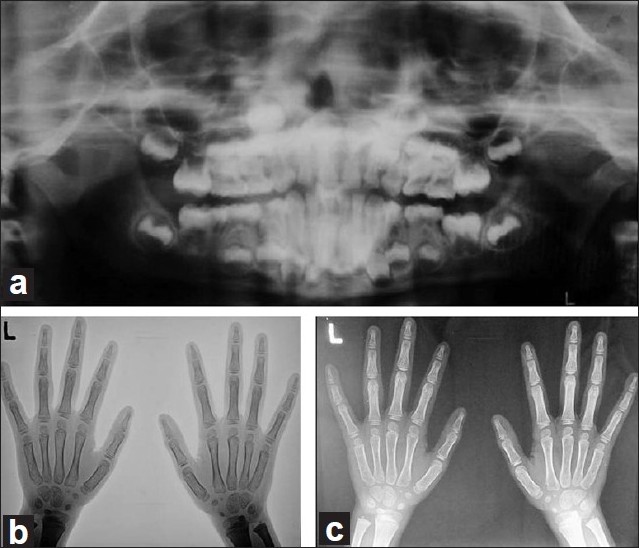 |
| Figure 4: Radiographs of the elder boy. (a) OPG revealing mixed dentition and mild to moderate alveolar bone loss. (b) Negative image of hand wrist radiograph. (c) Hand wrist radiographs showing generalized osteopenia along with acroosteolysis of the right index and little finger |
Cultures for Actinobacillus actinomycetamcomitans could not be performed due to unavailability of the investigation in and around our institution.
As HMS and Papillon lefevre syndrome (PLS) results from the same allelic mutation of cathepsin C gene, the treatment followed for HMS was the same as for Papillon lefevre syndrome given by Lee et al.[2]
Before initiation of the treatment both the siblings underwent baseline investigations in the form of full blood count, liver function tests, electrolytes, fasting cholesterol and triglycerides which did not reveal any abnormality.
Finally both the patients were prescribed a dose of acitretin 10 mg orally, along with systemic antibiotics in the form of Cotrimoxazole (200:40) and topical keratolytics which included 6% coal tar, sulphur and salicyclic acid (salytar) for a period of one year.
Within four weeks of the initiation of the treatment there was marked regression of the palmoplantar keratoderma in both siblings. Initially both patients were put on acetretin 10 mg once daily for two days of every three for eight weeks (i.e. the patient has to skip the medication on the third day) and then every alternate day for next eight weeks and then once in three days for another eight weeks. Both the patients were followed on the same maintenance dose for next six months and reviewed monthly. The baseline investigations were repeated every six months.
One year after initiating the treatment with acetretin, the skin of both the patients remained almost lesion free with exacerbations during the winters. The periodontal health also improved markedly as the teeth remained immobile with decreased periodontal pocket depths after one year of treatment. The response to treatment was better in the elder boy when compared to the younger girl.
Discussion
Haim Munk syndrome was first described by Haim and Munk in 1965. HMS is a rare syndrome reported in migrant Jews from Kerala to Israel in 1965 and has also been described among descendants of a Jewish isolate originally from Cochin, India. [3],[4] To the best of knowledge this is supposed to be one of the few case report of HMS from Northern India.
Parental consanguinity is the main etiological factor in HMS. [3] Also, a complex, autosomal recessive pattern of inheritance with phenotypic influences from a closely linked modifying locus has been hypothesised for HMS. Mutations of the cathepsin C gene exon 6 CTSC codon have been identified as the underlying genetic defect in HMS. [3],[5] Owing to the low socio economic status of the patient and the lack of facilities in our institute, genetic analysis for cathepsin C gene could not be done in our patients. The lack of functional cathepsin C in HMS may be associated with a reduced host response against microorganisms in dental plaques and possibly at other sites. A possible role of A. actinomycetemcomitans has been reported in HMS periodontitis. [2]
Cathepsin C (dipeptidyl-peptidase I) is a lysosomal cysteine proteinase that is important in intracellular degradation of proteins and coordinator for activation of serine proteinases in immune inflammatory cells, particularly polymorphonuclear leucocytes. Cathepsin C is normally expressed in palmar, plantar, and gingival epithelium, the tissues mainly affected by HMS. In addition to being expressed in skin, cathepsin C is present in large amounts in osteoclasts hence has a role in bone modelling and remodelling. The junctional epithelium and palmoplantar epithelium share common developmental characteristics, and hence pathological effects of cathepsin C mutations are manifested in these tissues. [3],[6]
In addition to congenital palmoplantar keratosis and progressive early onset periodontal destruction, other clinical findings of HMS includes recurrent pyogenic skin infections, acroosteolysis, atrophic changes of the nails (onychogryposis), arachnodactyly, and a peculiar radiographic deformity of the fingers consisting of tapered, pointed phalangeal ends, claw-like volar curve and pes planus. [3],[7],[8] All of these features, present with the elder boy of our case report were consistent with the diagnosis.
HMS should be differentiated from PLS as both are the allelic mutations of the Cathepsin C gene. The presence of arachnodactyly, acroosteolysis, pes planus, and nail deformities are the primary clinical findings that differentiate between HMS and PLS. The skin manifestations, bone and finger deformities in HMS are more severe and extensive while the periodontal involvement in HMS is reported to be less severely affected than in PLS. [3]
A multidisciplinary approach involving the dermatologist, paediatrician and paediatric dentist is important for the overall care of the patient with HMS and PLS. Good dental care and the use of prophylactic antibiotics aims to minimize periodontitis and the loss of teeth. Eradication of A. actinomycetemcomitans has been shown to play a role in the successful treatment of periodontitis, and Cotrimoxazole has been demonstrated to be an effective drug against this organism [2]
With regard to the long-term safety of retinoid treatment it was demonstrated that it is a safe and effective treatment in children with inherited keratinization disorders. Retinoid treatment may cause skeletal abnormalities in children including osteoporosis, periosteal plucking, slender long bones and premature epiphyseal closure. [2] In our patients none of the side effects of retinoids were observed in the bony as well as in periodontal condition during one year follow up.
The benefit of this medication to our patients, who are now able to live a normal life, would appear to outweigh the risks when one considers the evidence to date presented in the literature.
| 1. |
Sherak NB, Thomson G. Gale Encyclopedia of Genetic Disorders Part II. Available from: http://www.healthline.com/galecontent/haim-munk-syndrome-1/3 . [cited in 2005].
[Google Scholar]
|
| 2. |
Lee MR, Wong LC, Fischer GO. Papillon Lefèvre syndrome treated with acitretin. Australasian J Dermatol 2005;46:199-201.
[Google Scholar]
|
| 3. |
Hart TC, Hart PS, Michalec MD. Haim-Munk syndrome and Papillon-Lefèvre syndrome are allelic mutations in cathepsin C. J Med Genet 2000;37:88-94.
[Google Scholar]
|
| 4. |
Gorlin RJ. Of palms, soles, and gums. J Med Genet 2000;37:81-2.
[Google Scholar]
|
| 5. |
Selvaraju V, Markandaya M, Siva Prasad PV, Sathyan P, Sethuraman G, Srivastava SC, et al. Mutation analysis of the cathepsin C gene in Indian families with Papillon-Lefèvre syndrome. BMC Med Genet 2003;4:1-8.
[Google Scholar]
|
| 6. |
Cury VF ,Gomez RF, Costa JE, Friedman E, Boson W, De Marco L. A homozygous cathepsin C mutation associated with Haim-Munk syndrome. Br J Dermatol 2005;152:353-6.
[Google Scholar]
|
| 7. |
Puliyel JM, Iyer KS. A syndrome of keratosis palmo-plantaris congenita, pes planus, onychogryphosis, periodontosis, arachnodactyly and a peculiar acro-osteolysis. Br J Dermatol 1986;115:243-8.
[Google Scholar]
|
| 8. |
Janjua SA, Iftikhar N, Hussain I, Khachemoune A. Dermatologic, periodontal, and skeletal manifestations of Haim-Munk syndrome in two siblings. J Am Acad Dermatol 2008;58:339-44.
[Google Scholar]
|
Fulltext Views
4,488
PDF downloads
3,117





