Translate this page into:
High serum total IgE levels correlate with urticarial lesions and IgE deposition in perilesional skin of bullous pemphigoid patients: An observational study
Corresponding author: Dr. Giang Pham Ngan, Department of Dermatology, Hanoi Medical University, Ton That Tung, Hanoi, Vietnam. giangsoc@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ngan GP, Nguyen VTH, Huu DL. High serum total IgE levels correlate with urticarial lesions and IgE deposition in perilesional skin of bullous pemphigoid patients: An observational study. Indian J Dermatol Venereol Leprol. 2025;204:204-9. doi: 10.25259/IJDVL_610_2023
Abstract
Background
In the pathophysiology of bullous pemphigoid, besides IgG, there has been evidence that supports the role of IgE antibodies. However, there have been no studies to evaluate total serum IgE levels or detect IgE deposits in the skin of Vietnamese patients.
Aim
To analyse the association between IgE levels in the serum and disease severity as well as eosinophils and IgE basement membrane zone (BMZ) deposition in Vietnamese bullous pemphigoid patients.
Methods
A single-centre observational research on 35 newly diagnosed and untreated bullous pemphigoid patients. Total serum IgE levels were analysed using enzyme‐linked immunosorbent assay (ELISA). For controls, we collected sera of 30 pemphigus patients and 30 elderly patients with pruritus. Perilesional skin biopsies underwent direct immunofluorescence (DIF) staining, with biopsies of pemphigus patients as controls.
Results
Elevated total serum IgE was observed in 60% of bullous pemphigoid patients, the percentage in the pemphigus group and pruritus group was 20% and 40%, respectively. The mean total serum IgE level among the bullous pemphigoid group was higher than that of the pemphigus group (123.3 ± 102.4 IU/mL vs. 64.3 ± 45.1 IU/mL, p = 0.010). Total serum IgE levels of bullous pemphigoid patients correlated with higher eosinophil counts (r = 0.61; p = 0.018) and urticaria/erythema (U/E) Bullous Pemphigoid Disease Area Index (BPDAI) score (r = 0.50; p = 0.035). Among 35 bullous pemphigoid patients, 5 patients showed positive IgE DIF staining, accounting for 14.3%. Higher serum IgE levels correlated with the deposition of IgE in patients’ perilesional skin (p = 0.037).
Limitations
Due to the rarity of bullous pemphigoid, the effect of the COVID-19 pandemic, and self-treatment issues in Vietnam, we could not recruit a larger number of participants.
Conclusions
Total serum IgE values correlated with urticarial lesions and IgE deposition in perilesional skin of Vietnamese bullous pemphigoid patients. IgE autoantibodies present in the skin of bullous pemphigoid patients support the role of IgE in bullous pemphigoid pathogenesis.
Keywords
BPDAI
direct immunofluorescence
eosinophil
IgE
pemphigoid
Introduction
Bullous pemphigoid is a type of autoimmune bullous disease.1,2 Although its prevalence in the population is relatively low, this disease can cause many serious complications.2 There is increased mortality in bullous pemphigoid patients with prolonged illnesses, especially in immunocompromised or debilitated patients.3 Moreover, corticosteroids are commonly used in the treatment of this disease but they can cause various side effects.4 Therefore, it is important to diagnose pemphigoid early and provide timely treatment. Also, studying the pathogenesis and prognostic factors can help improve the outcome of pemphigoid.
The current diagnostic criteria of pemphigoid include the identification of autoantibodies in both the skin and serum of patients, among which the most common are IgG autoantibodies.5,6 Besides, previous studies reported increased total serum IgE levels in pemphigoid patients, as well as positive results of specific IgE autoantibodies in the serum and IgE deposition along basement membrane zone (BMZ) on direct immunofluorescence (DIF) staining in patients with pemphigoid.7–13 This finding led to a novel treatment therapy for pemphigoid disease, which is omalizumab. There is increasing evidence for the effectiveness and safety profile of omalizumab in recalcitrant pemphigoid.14 Currently in Vietnam, there has not been any study on the total serum IgE level in pemphigoid patients. Therefore, this study was conducted to analyse the association between IgE levels in the serum and disease severity as well as eosinophil and IgE deposition in Vietnamese bullous pemphigoid patients at a dermatology centre from 2021 to 2022.
Methods
Primary objectives: To investigate the correlation between total serum IgE level and the Bullous Pemphigoid Disease Area Index (BPDAI) score and urticaria/erythema (U/E) BPDAI score.
Secondary objectives: To investigate the correlation between total serum IgE level and DIF IgE staining as well as eosinophil counts.
Study populations: In this descriptive cross-sectional study, newly diagnosed and untreated bullous pemphigoid patients at a dermatology centre from July 2021 to September 2022 were recruited in the study. While patients with conditions that affect IgE levels such as asthma, atopic dermatitis or allergic rhinitis and patients with other skin diseases that cause pruritus, such as acute urticaria, scabies, etc. were excluded from the study. In addition, we also excluded patients who took systemic corticosteroids or H1 antihistamines within 3 weeks previous to the study.
Controls: Thirty pemphigus patients and 30 elderly patients with pruritus were included as controls. All were newly diagnosed and treatment naïve. The pruritus group was excluded from non-bullous pemphigoid by negative indirect and direct IF.
Sample: To investigate the elevated rate of total serum IgE levels among bullous pemphigoid patients, the WHO estimation formula of the descriptive study was applied:
n: sample size; p: anticipated population proportion of patients with elevated total serum IgE level; Z1–α/2: Confidence interval (α = 0.05): 95%, Z = 1.96; ε: relative precision, ε = 0.3
In this study, we chose p = 59.3%7 as the anticipated population proportion of bullous pemphigoid patients with elevated total serum IgE levels. When applied to the formula, n = 30.
From July 2021 to September 2022, 35 newly diagnosed bullous pemphigoid patients at the dermatology centre participated in the study.
Study procedure
Patients were diagnosed with bullous pemphigoid if three diagnostic criteria were met: (i) consistent clinical features, (ii) positive linear IgG on DIF and (iii) positive salt-split skin IgG DIF staining on the roof of blister.5,6 BPDAI score was used to assess the disease severity.
Total-IgE ELISA Test Kit was used to determine total serum IgE levels (normal IgE range < 100 IU/mL). Eosinophil counts were analysed on the same day when serum samples were taken for IgE determination (normal range of eosinophil count: 0.0–0.8 G/L). For DIF staining of IgE, after the procedure was done, skin biopsy specimens were stored at –80oC. Incubation was performed overnight at 4oC. The study was conducted as described in the STROBE flowchart [Figure 1].15

- STROBE flowchart (STROBE: Strengthening the Report of Observational Studies in Epidemiology).
Statistical analysis
SPSS 20.0 software was used for statistical analysis. Chi-square tests, Fisher’s exact tests, Mann–Whitney U-test and Spearman’s test (r) were used to determine the statistical significance and dependence between variables.
Results
Among 35 newly diagnosed bullous pemphigoid patients, 25 were men, accounting for 71.4%, while 10 were women, comprising 28.6% of the total cases. The average age of the patients was 73.2 ± 15.5 years. Elevated eosinophil counts were observed in 19 of 35 bullous pemphigoid patients (54.3%), in four patients (13.3%) of the pemphigus group and in 11 patients (36.6%) of the pruritus group. The proportion of eosinophilia was significantly higher in the bullous pemphigoid group compared to the pemphigus group, p = 0.001. Total IgE was elevated in 21 (60%) bullous pemphigoid patients, and the percentages for pemphigus controls and elderly controls were 6 (20%) and 12 (40%), respectively. The proportion of elevated IgE was significantly higher in the bullous pemphigoid group compared to the pemphigus group, p = 0.001. In the bullous pemphigoid group, the mean total serum IgE level was 123.3 ± 102.4 IU/mL, which was higher than that of the pemphigus group (64.3 ± 45.1 IU/mL, p =0.010). [Table 1].
| Study group | Control group | Elderly subjects with pruritus n = 30 | |
|---|---|---|---|
| Pemphigoid n = 35 | Pemphigus n = 30 | ||
| Mean age (Years) | 73.2 ± 15.5 | 60.8 ± 8.9 | 72.7 ± 7.5 |
| Gender, n (%) | |||
| Male, n (%) | 25 (71.4) | 7 (23.3) | 21 (70.0) |
| Female, n (%) | 10 (28.6) | 23 (76.7) | 9 (30.0) |
| Eosinophilia, n (%) | 19 (54.3) | 4 (13.3) | 11 (36.6) |
| Elevated total IgE, n (%) | 21 (60) | 6 (20.0) | 12 (40.0) |
| Mean total IgE level (M, SD) (IU/mL) *, ** | 123.3 ± 102.4 | 64.3 ± 45.1 | 80.9 ± 36.4 |
| DIF positive for linear IgE, n (%) | 5 (14.3) | 0 |
Serum IgE levels in pemphigoid patients correlated with eosinophil counts, with correlation coefficient r = 0.61; p = 0.018 [Figure 2]. Higher total serum IgE level was not significantly associated with a higher BPDAI rating (r = 0.37; p = 0.082) [Figure 3]. However, the score of urticaria and erythema (U/E) in the BPDAI scale had a positive correlation with the total serum IgE levels with the correlation coefficient r = 0.50 (p = 0.035). [Figure 4].
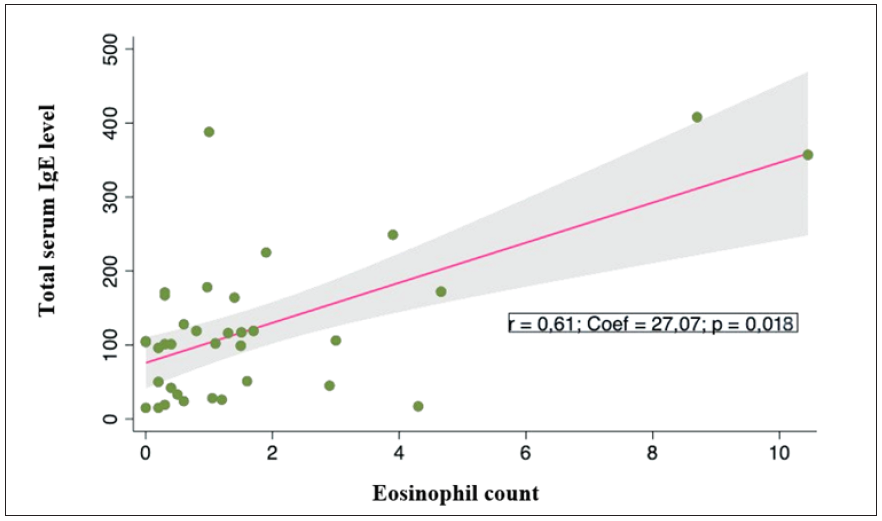
- Correlation between total serum IgE levels (IU/mL) and peripheral eosinophil counts (G/L) and bullous pemphigoid patients.
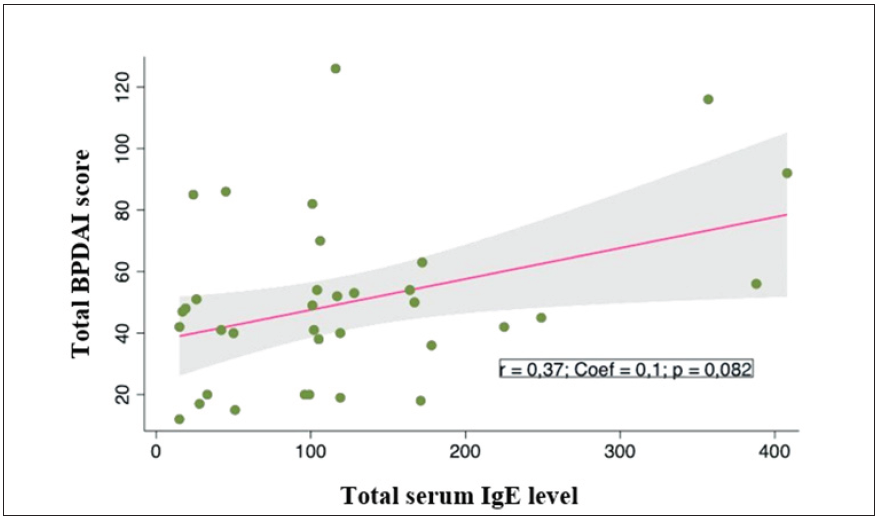
- Correlation between total serum IgE levels (UI/mL) and total BPDAI score (point) in bullous pemphigoid patients.
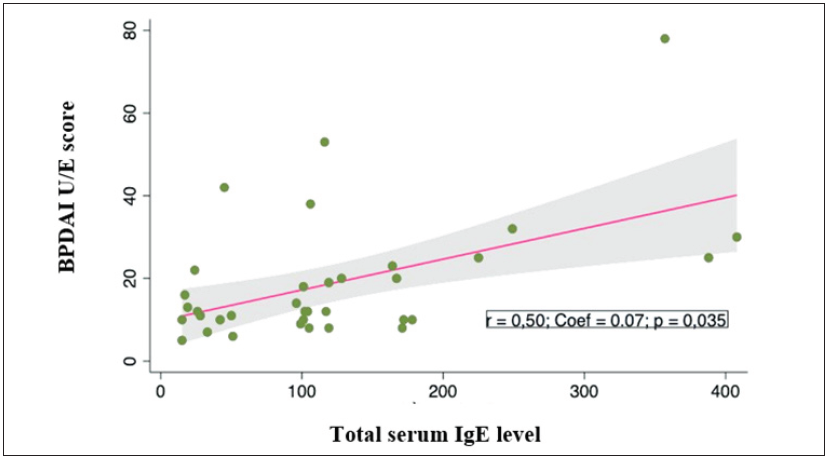
- Correlation between total serum IgE levels (UI/mL) and U/E BPDAI score (point) in bullous pemphigoid patients.
IgE linear deposits along the BMZ were observed in five cases (14.3%) [Figure 5]. Total serum IgE level associated with IgE deposition along the BMZ (p = 0.037). Besides, positive IgE stainings were significantly associated with higher eosinophil counts (p = 0.021) and BPDAI scores (p = 0.005) [Table 2].
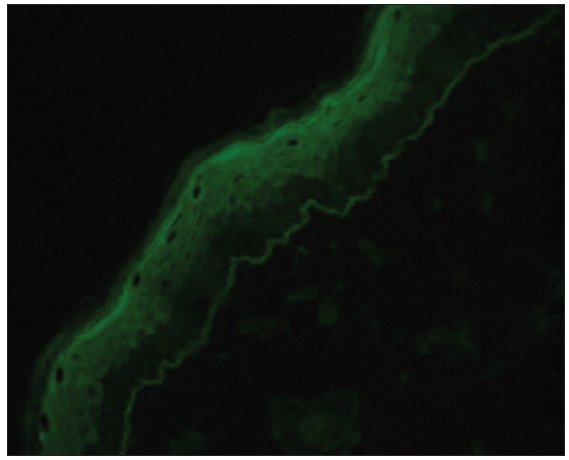
- Linear deposits of IgE found by direct immunofluorescence microscopy (400x).
| IgE DIF staining | p | ||
|---|---|---|---|
| Positive n=5 | Negative n=30 | ||
| Mean total serum IgE level (IU/mL) | 233.8 ± 62.4 | 104.9 ± 15.4 | 0.037* |
| Eosinophil counts (G/L) | 3.8 ± 3.1 | 1.3 ± 1.5 | 0.021* |
| BPDAI | 83.6 ± 27.0 | 44.1 ± 23.0 | 0.005* |
| U/E BPDAI | 29.4 ± 18.7 | 17.1 ± 14.0 | 0.113* |
*Mann–Whitney test, bold value just means it’s <0.05 (p < 0.05), hence it’s statically significant.
Discussion
Eosinophilia was observed in 54.3% of patients, similar to the findings of Lamberts et al.7 and Kridin.16 Eosinophilia and eosinophil infiltration of the dermis in pemphigoid patients are explained by the role of this leukocyte in the pathogenesis of the disease.17 Messingham et al.18 also reported that there may be a certain correlation between eosinophil levels and the severity of pemphigoid.
The mean serum IgE level in the bullous pemphigoid group was 123.3 ± 102.4 IU/mL. The result in Lamberts’ study was similar to our study, which used the same enzyme‐linked immunosorbent assay (ELISA) technique.7 The elevation of serum IgE levels could be explained by the hypothesised IgE autoantibodies’ role in the pathogenesis of the disease.7,8
Our study found a significant correlation between eosinophil counts and total serum IgE levels, which was consistent with Messingham’s study.18 In pemphigoid patients, eosinophils increased expression of FcεRI, which is a receptor with a high affinity for IgE.18
There was a significant correlation between the serum IgE levels and U/E BPDAI score. This result was similar to van Beek’s study.8 Cozzani showed that although the total serum IgE levels were higher in patients with (mainly) bullae compared to patients with (mainly) urticarial lesions, the IgE levels were unrelated to disease severity,19 similar to our findings. This may be explained by the role of IgG and complement autoantibodies in the pathogenesis of pemphigoid, in addition to IgE autoantibodies.
Linear IgE deposits along the BMZ were observed in five cases (14.3%). The positive rates varied widely: 0%, 3%, 7%, 18%, 25% and 44% as reported by several previous studies.7,9–13 These varying results can be attributed to different staining methods and biopsy sites. In our study, we observed that the group with positive staining had statistically significantly higher levels of circulating IgE and a higher number of eosinophils compared to the group with negative IgE staining (p < 0.05). The IgE DIF positive group also had a higher BPDAI score. However, U/E BPDAI scores showed no statistical difference between the two groups, which was similar to a study by Kamata in 2020.11
Limitations
At the beginning of our study on pemphigoid, we were only able to recruit 42 patients due to the rarity of the condition and the impact of the COVID-19 pandemic. However, six patients were excluded from the study as they self-treated and bought over-the-counter medicines, which is common in Vietnam. Nevertheless, this sample size was not smaller than previous studies conducted in the same duration in this centre before the pandemic. Due to the time limit and probable misdiagnosis, we could not recruit any non-bullous pemphigoid patients. Moreover, our study was the very first step to assess the total non-specific IgE levels, and we hope to conduct future studies on IgE-specific NC16A of BP180 and BP230.
Conclusion
Among 35 bullous pemphigoid patients, total serum IgE levels were elevated in 60% of patients. Higher serum IgE levels correlated with both eosinophil counts and U/E BPDAI score. DIF of pemphigoid patients for IgEdeposits showed a positivity rate of 14.3%. Linear deposits of IgE in the BMZ in pemphigoid patients correlated with increased serum IgE levels.
Ethical approval
The Ethical Review Board of the National Hospital of Dermatology and Venereology approved our research proposal (Ethics approval number: 371/HĐĐĐ – BVDLTW, Study registration number: 5306/QĐ-ĐHYHN).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Diagnosis of autoimmune bullous diseases: Autoimmune bullous diseases. J Dtsch Dermatol Ges. 2018;16:1077-91.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of bullous pemphigoid and mortality of patients with bullous pemphigoid in Olmsted County, Minnesota, 1960 through 2009. J Am Acad Dermatol. 2014;71:92-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bullous pemphigoid: Corticosteroid treatment and adverse effects in long-term care patients. Consult Pharm. 2013;28:455-62.
- [CrossRef] [PubMed] [Google Scholar]
- Vesiculobullous disorders. In: Fitzpatricks dermatology (9th ed). New York: McGraw-Hill Education; 2019. p. :909-1035.
- [Google Scholar]
- Diagnosis and classification of pemphigus and bullous pemphigoid. Autoimmun Rev. 2014;13:477-81.
- [CrossRef] [PubMed] [Google Scholar]
- IgE autoantibodies in serum and skin of non‐bullous and bullous pemphigoid patients. J Eur Acad Dermatol Venereol. 2021;35:973-80.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Correlation of serum levels of IgE autoantibodies against BP180 with bullous pemphigoid disease activity. JAMA Dermatol. 2017;153:30.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of linear IgE deposits in bullous pemphigoid and mucous membrane pemphigoid: A useful clue for diagnosis: Linear IgE deposits in BP and MMP. Br J Dermatol. 2011;165:1133-7.
- [CrossRef] [PubMed] [Google Scholar]
- In vivo analysis of IgE autoantibodies in bullous pemphigoid: A study of 100 cases. J Dermatol Sci. 2015;78:21-5.
- [CrossRef] [PubMed] [Google Scholar]
- Basement membrane zone IgE deposition is associated with bullous pemphigoid disease severity and treatment results. Br J Dermatol. 2020;182:1221-7.
- [CrossRef] [PubMed] [Google Scholar]
- IgE autoreactivity in bullous pemphigoid: Eosinophils and mast cells as major targets of pathogenic immune reactants. Br J Dermatol. 2017;177:1644-53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J Invest Dermatol. 2003;120:784-8.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab and omalizumab for the treatment of bullous pemphigoid: A systematic review of the literature. Am J Clin Dermatol. 2019;20:209-16.
- [CrossRef] [PubMed] [Google Scholar]
- The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-9.
- [CrossRef] [PubMed] [Google Scholar]
- Peripheral eosinophilia in bullous pemphigoid: Prevalence and influence on the clinical manifestation. Br J Dermatol. 2018;179:1141-7.
- [CrossRef] [PubMed] [Google Scholar]
- The role of eosinophils in bullous pemphigoid: A developing model of eosinophil pathogenicity in mucocutaneous disease. Front Med (Lausanne). 2018;5:201.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Human eosinophils express the high affinity IgE receptor, FcεRI, in bullous pemphigoid. PLoS One. 2014;9:e107725.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bullous pemphigoid in Liguria: A 2-year survey. J Eur Acad Dermatol Venereol. 2001;15:317-9.
- [CrossRef] [PubMed] [Google Scholar]







