Translate this page into:
Hypereosinophilic syndrome masquerading as acrodermatitis enteropathica
Correspondence Address:
P Sivasankri
Department of Dermatology Venereology and Leprosy, Rajiv Gandhi Government General Hospital and Madras Medical College, Chennai, Tamil Nadu
India
| How to cite this article: Sivasankri P, Ramesh A, Sampath V. Hypereosinophilic syndrome masquerading as acrodermatitis enteropathica. Indian J Dermatol Venereol Leprol 2019;85:418-422 |
Sir,
Hypereosinophilic syndrome most commonly involves the heart, central nervous system, lungs and the skin. Gastrointestinal tract involvement is very rare. We report a case that presented with skin manifestations of acquired acrodermatitis enteropathica secondary to hypereosinophilic syndrome of the gastrointestinal tract.
A 36-year-old unmarried gentleman presented to Rajiv Gandhi Government General Hospital, Chennai, Tamil Nadu, India with multiple episodes of erosive desquamating plaques all over the body, particularly on the flexures and extremities, with intense itching and chronic diarrhea for the last 10 years. On examination, he was short (height: 140 cm), had sparse hair in the axillary, pubic, beard and mustache area but testis and scrotum were normal. He had generalized xerosis, erosive desquamating plaques on the trunk and extremities, predominantly on the flexures, with sparing of the face, palms and soles [Figure - 1]a, [Figure - 1]b, [Figure - 1]c. He also had angular cheilitis, perianal depigmentation with mild erythema, and inguinal and cervical lymphadenopathy.
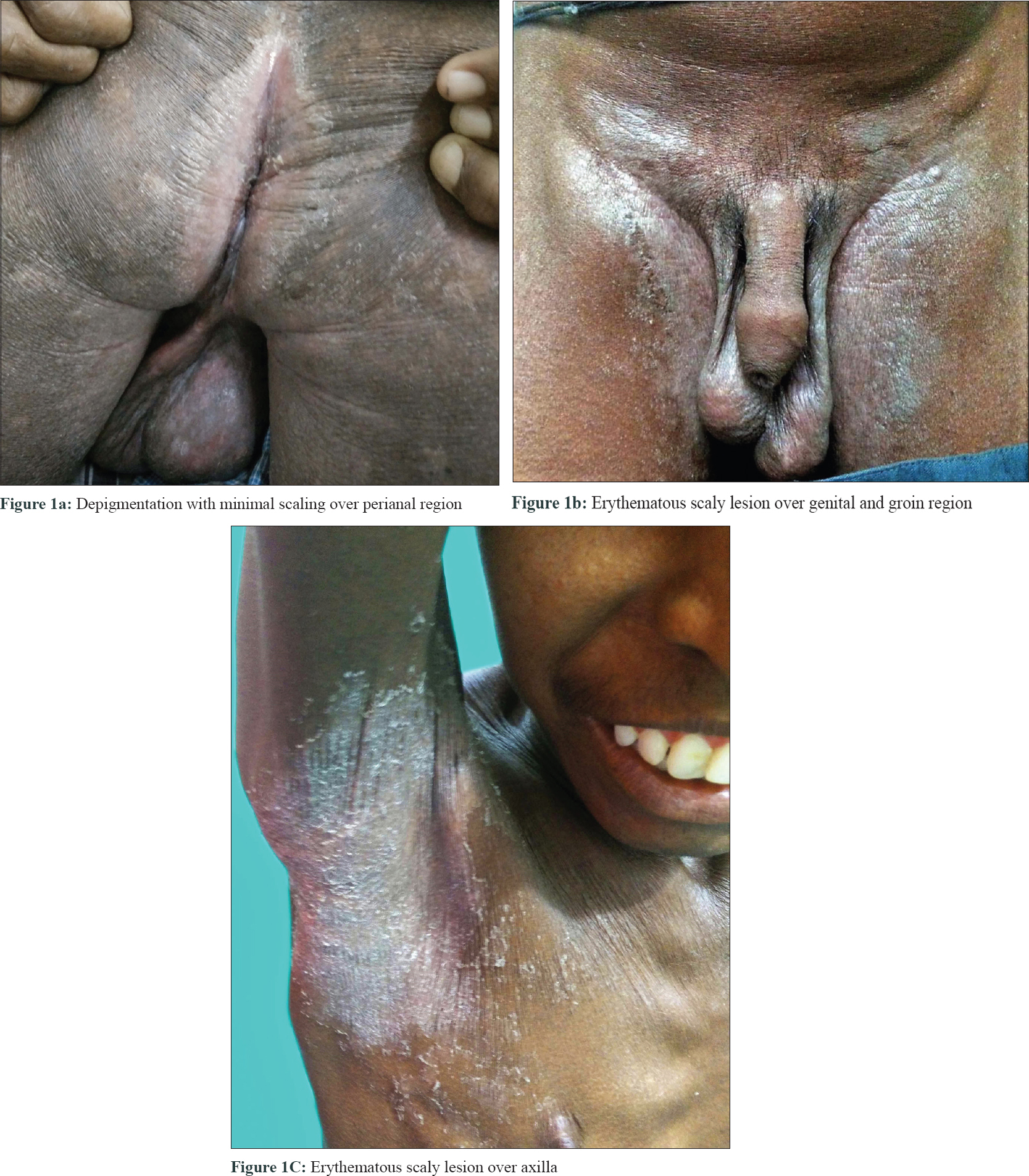 |
| Figure 1: |
His routine blood investigations revealed anemia (hemoglobin: 8 g/dl), thrombocytosis (platelet: 789,000 cells/cu.mm), leukocytosis (16,300 cells/cu.mm), eosinophilia (2867/cu.mm) and increased lactate dehydrogenase. The serum zinc level was 29 μg/dl (normal: 70–125 μg/dl), and serum alkaline phosphatase was 122 IU/l (normal: 30–140 IU/l). Histopathology of skin lesion showed hyperkeratosis, subcorneal vesicles, acanthosis, spongiosis and perivascular and peri-appendageal inflammatory infiltrate composed of lymphocytes, neutrophils and some eosinophils, consistent with psoriasiform dermatitis [Figure - 2]. In view of the clinical picture, hypozincemia and histologic changes, diagnosis of acquired acrodermatitis enteropathica was made, and the patient was started on oral zinc 3 mg/kg/day and emollients, but there was no improvement of the skin symptoms even after 1 month. At the same time, the patient was being investigated for diarrhea and anemia; his bone marrow biopsy revealed marrow hyperplasia with increased eosinophilic precursors. His colonic biopsy revealed eosinophilic colitis (eosinophil count >25 per high power field) [Figure - 3] and esophageal biopsy was reported to be eosinophilic esophagitis (eosinophil count >7 per high power field) [Figure - 4]. Fine-needle aspiration cytology of lymph nodes showed reactive lymphadenitis. Repeat absolute eosinophil count done at this point was 3456/cu.mm, after 8 months of initial investigation. His serum immunoglobulin E level was found to be increased (5478 IU/ml). Hence, the final diagnosis was revised to hypereosinophilic syndrome.
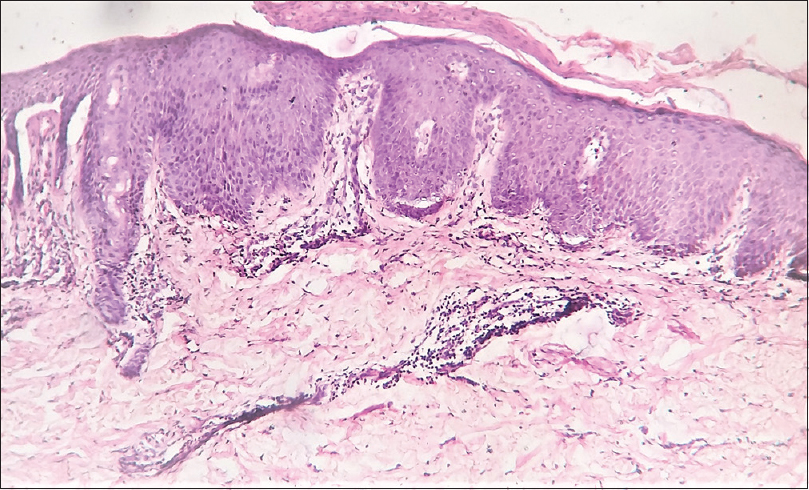 |
| Figure 2: Skin biopsy showing hyperkeratosis, parakeratosis, subcorneal vesicle, psoriasiform acanthosis, perivascular infiltrate of mononuclear cells (hematoxylin and eosin, × 100) |
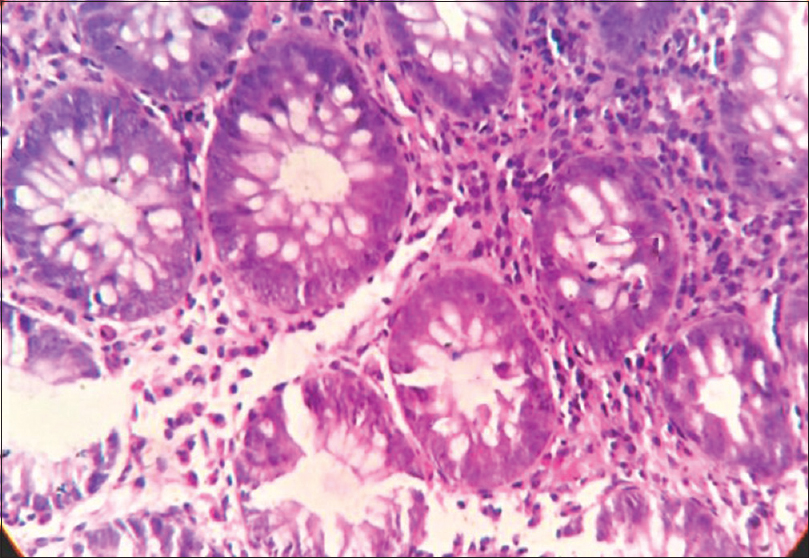 |
| Figure 3: Colon biopsy showing ulcerated colonic mucosa with focal areas of cryptitis. Lamina propria showing intense eosinophilic infiltrate (>25 per high power field) (hematoxylin and eosin, ×400) |
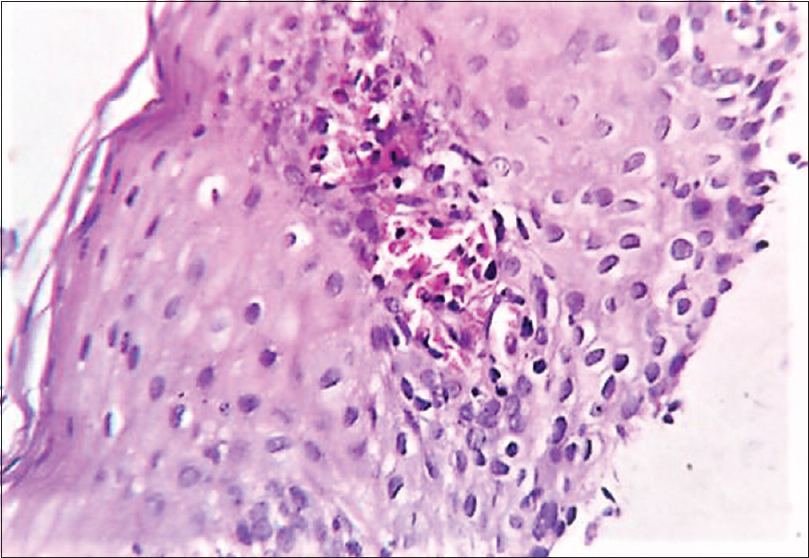 |
| Figure 4: Esophageal biopsy showing ulcerated stratified squamous epithelium with intraepithelial inflammatory infiltrate, predominantly eosinophils (>7 per high power field). Focus of eosinophilic microabscess seen (hematoxylin and eosin, ×400) |
Investigations to screen other system involvement such as echocardiography, magnetic resonance imaging brain, computed tomography chest were all normal. Serum B12 level was normal (239 pg/ml). Viral markers (hepatitis B, C, HIV) and rheumatoid factor were negative. Investigations to rule out secondary causes of eosinophilia such as stool for ova-cyst, blood culture for bacteria, antinuclear antibodies, C-antineutrophil cytoplasmic antibody and P-antineutrophil cytoplasmic antibody were negative. Serum tryptase and interleukin-5 levels were not done because of lack of facilities. The patient was then started on oral prednisolone 1 mg/kg/day in addition to zinc acetate 3 mg/kg/day and showed dramatic clinical improvement within a month [Figure - 5]a, [Figure - 5]b, [Figure - 5]c. His absolute eosinophil count after 2 months of systemic steroids decreased to 130/mm[3] and the steroid was then gradually tapered.
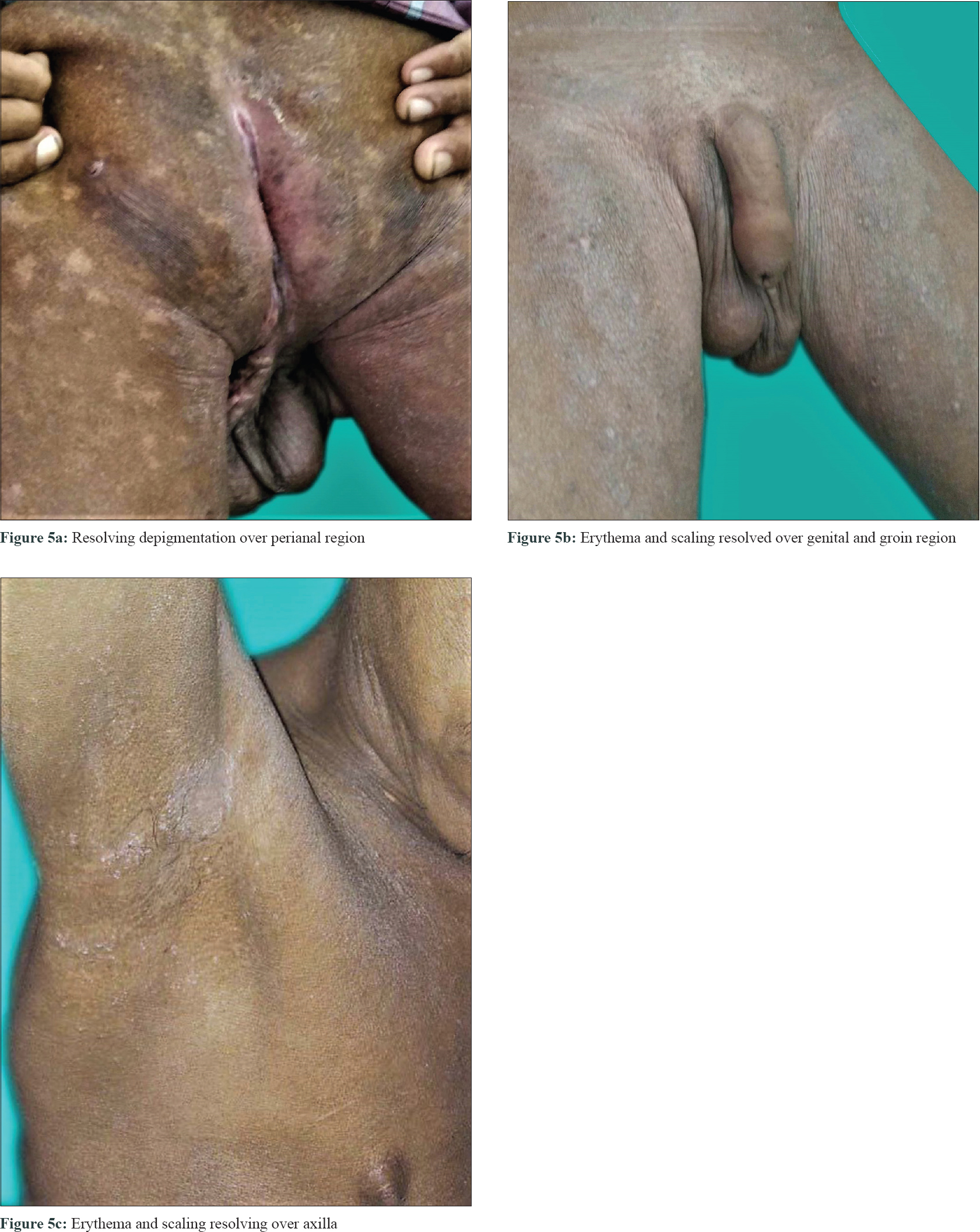 |
| Figure 5: |
Hypereosinophilic syndrome was first described in 1968, by Hardy and Anderson. It manifests with varied clinical presentation and so, is essentially a diagnosis of exclusion. The revised diagnostic criteria of hypereosinophilic syndrome are:
- Blood eosinophilia >1500 eosinophils/mm[3] on at least two separate determinations or evidence of prominent tissue eosinophilia associated with symptoms and marked blood eosinophilia.
- Exclusion of secondary causes of eosinophilia such as parasitic or viral infections, allergic diseases, drug or chemical-induced eosinophilia, hypoadrenalism and neoplasms.[1]
The tissues most vulnerable and most frequently affected by eosinophils in decreasing order of frequency are the heart (~60%), skin, nervous system, respiratory and gastrointestinal tract (~20%).[2]
There are three forms of the disease: myeloproliferative, lymphocytic and familial. Myeloproliferative form associated with FIP1L1-PDGFRA mutation is more common in men. This usually presents with fever, weight loss, fatigue, malaise, skin lesions and hepatosplenomegaly and responds to imatinib. Cardiac disease is more frequent, and the patient usually dies within 2 years of presentation. Serum B12 levels and tryptase may be raised in the myeloproliferative form of hypereosinophilic syndrome. In our patient, serum B12 was normal and tryptase was not done. Lymphocytic form presents with pruritus, eczema, erythroderma, urticaria, angioedema and lymphadenopathy. Lymphocytic hypereosinophilic syndrome follows a more benign course.[3] There is often a raised serum immunoglobulin E level. Our patient had similar clinical feature and fits into this form of hypereosinophilic syndrome.
Eosinophilic gastroenteritis affects the entire gastrointestinal tract.[4] It is diagnosed by the presenting gastrointestinal symptoms, pathological eosinophilic infiltration of the gastrointestinal tract, no eosinophilic involvement of organs outside the gastrointestinal tract and no parasite infestation.[4] Abdominal pain and diarrhea with malabsorption have been described, explaining the underlying zinc deficiency in our case.
In hypereosinophilic syndrome, eosinophil count tends to fluctuate with the interleukin-5 levels and the symptoms worsen or get better depending on the eosinophil count. Our patient had an increase in absolute eosinophil count on the second occasion and there was corresponding worsening of the skin manifestations. Treatment includes systemic glucocorticoid 0.5–1 mg/kg/day, which is effective in reducing the eosinophil count, and antineoplastic drugs (cyclophosphamide, vincristine, cytarabine, etoposide) for slowing eosinophil production.[3],[5]
Acquired zinc deficiency has been reported in numerous conditions such as anorexia nervosa, alcoholism, inflammatory bowel disease, blind loop syndromes and total parenteral nutrition. Our patient had zinc deficiency secondary to eosinophilic gastroenteritis. Chronic zinc deficiency leads to growth retardation and hypogonadism which is evident in our case.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Simon HU, Rothenberg ME, Bochner BS, Weller PF, Wardlaw AJ, Wechsler ME, et al. Refining the definition of hypereosinophilic syndrome. J Allergy Clin Immunol 2010;126:45-9.
[Google Scholar]
|
| 2. |
Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis 2007;2:37.
[Google Scholar]
|
| 3. |
Leiferman KM, Peters MS. Eosinophils in cutaneous diseases. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolf K, editors. Fitzpatrick's Dermatology in General Medicine. 8th ed. The McGraw-Hill Companies Inc.; New York, ISBN: 978-0-07-171755-7, MHID: 0-07-171755-2, 2012. p. 387-91.
[Google Scholar]
|
| 4. |
Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 2004;113:11-28.
[Google Scholar]
|
| 5. |
Jeon YW, Hong SJ, Kim HJ, Han JP, Kim HK, Ko BM, et al. Ahypereosinophilic syndrome presenting as eosinophilic colitis. Clin Endosc 2012;45:444-7.
[Google Scholar]
|
Fulltext Views
4,006
PDF downloads
2,497





