Translate this page into:
Imatinib mesylate-induced pseudoporphyria in a patient with chronic myeloid leukemia
2 Department of Pathology, Rao Tula Ram Hospital, New Delhi, India
3 Department of Dermatology, Fortis Hospital, New Delhi, India
Correspondence Address:
Meenakshi Batrani
10, Aradhana Enclave, RK Puram, Sector 13, New Delhi - 110 066
India
| How to cite this article: Batrani M, Salhotra M, Kubba A, Agrawal M. Imatinib mesylate-induced pseudoporphyria in a patient with chronic myeloid leukemia. Indian J Dermatol Venereol Leprol 2016;82:727-729 |
Sir,
Imatinib mesylate is a tyrosine kinase inhibitor that targets the BCR-ABL, c-kit and platelet-derived growth factor receptors. It is the first-line agent in the treatment of Philadelphia chromosome-positive chronic myeloid leukemia and c-kit-positive gastrointestinal stromal tumors.[1]
Cutaneous reactions are one of the most common adverse effects of imatinib. The incidence of cutaneous reactions may vary from 7–88.9%, with maculopapular rash and superficial edema being the most frequent.[1] Rarely, porphyria cutanea tarda and pseudoporphyria have also been reported.[1]
A 75-year-old woman with chronic myeloid leukemia, on treatment for 11 months with imatinib 400 mg twice daily, presented with a 1 month history of blisters on the hands and feet. There was no history of photosensitivity. On examination, intact tense hemorrhagic bullae and erosions with scarring and milia formation were present on the dorsum of the hands and toes [Figure - 1]. A diagnosis of bullous pemphigoid was clinically suspected. A punch biopsy from the right little finger revealed a pauci-cellular subepidermal blister with festooning of dermal papillae into the blister cavity [Figure - 2]a. Periodic acid–Schiff staining revealed thickening of the small vessel walls in the papillary dermis [Figure - 2]b. Direct immunofluorescence demonstrated homogenous deposits of IgG, IgM and C3 in the vessel walls and a weak band at the dermoepidermal junction with IgG and C3 [Figure - 3]. The total urinary porphyrin level was within normal limits. Thus, with the clinical and laboratory findings, a diagnosis of imatinib-induced pseudoporphyria was made.
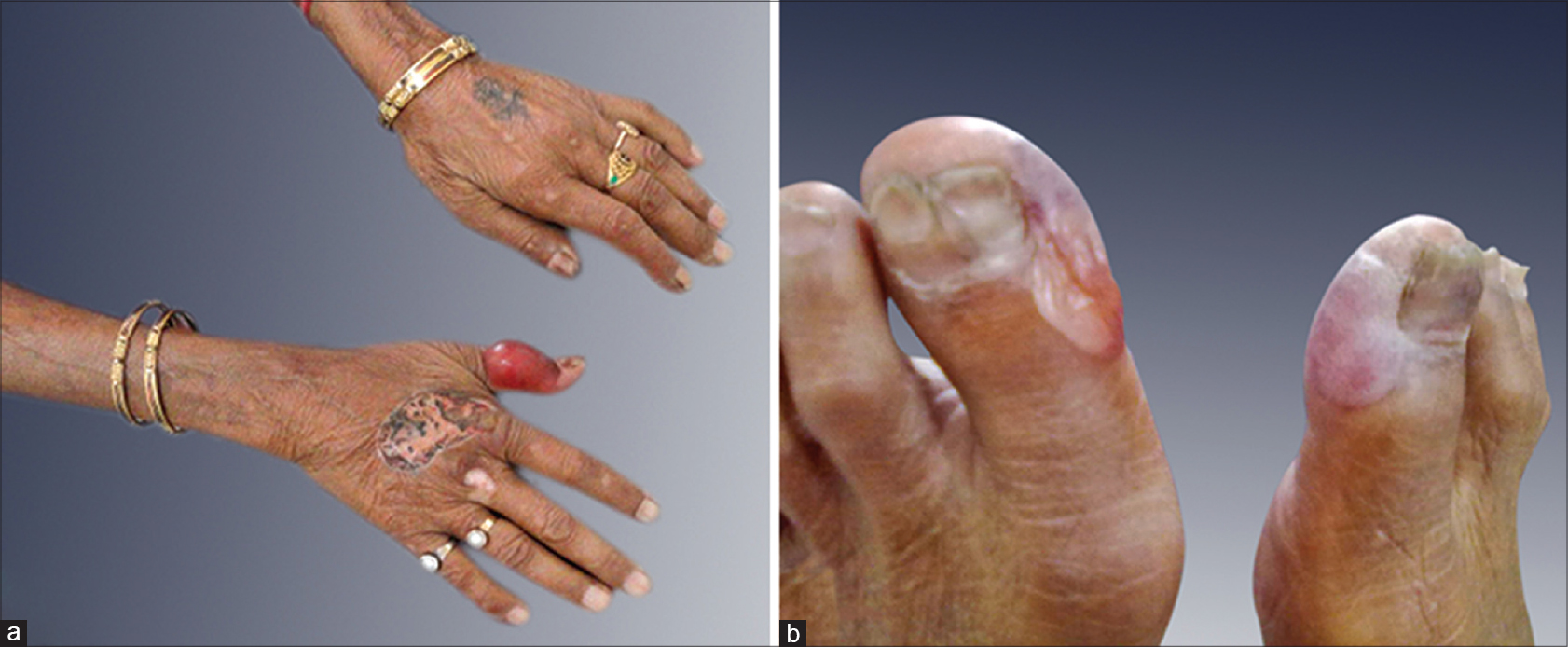 |
| Figure 1: Hemorrhagic tense bullae on the (a) hands and (b) feet with erosions and scarring |
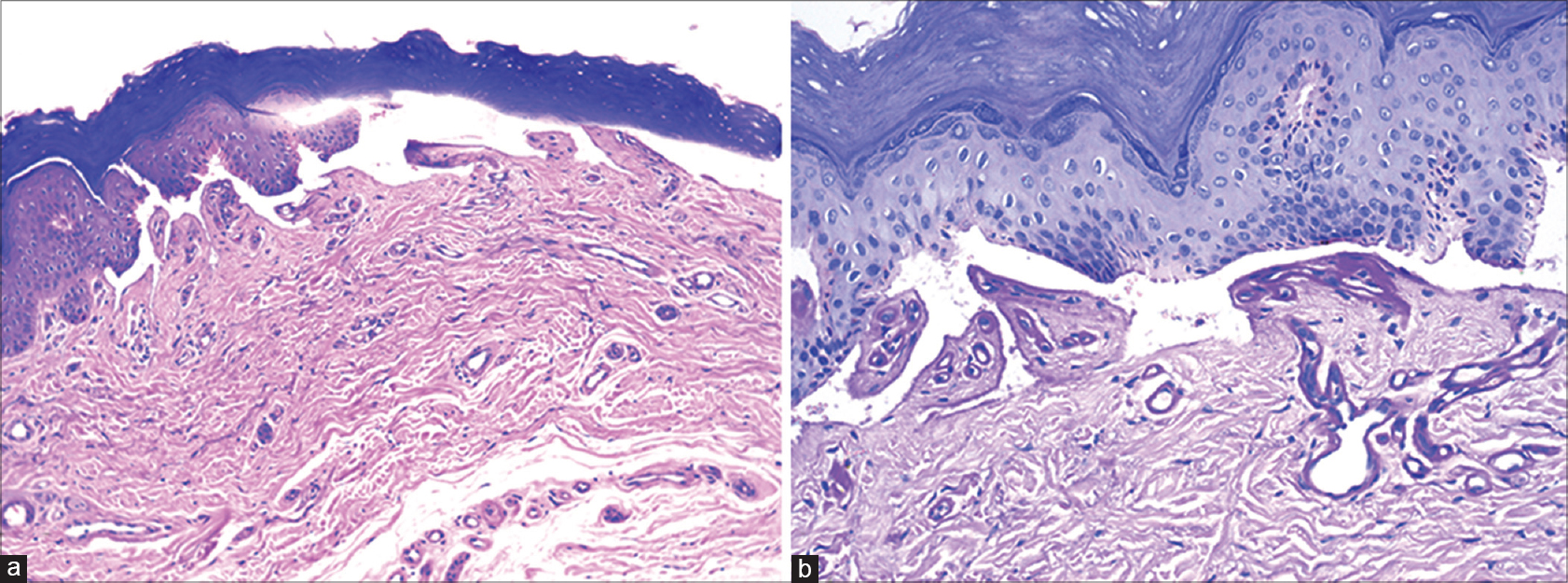 |
| Figure 2: (a) Pauci-cellular subepidermal blister with festooning of dermal papillae into the cavity (H and E, ×100). (b) Homogenous thickening of wall of small vessels in papillary dermis (Periodic acid–Schiff, ×200) |
 |
| Figure 3: Direct immunofluorescence demonstrating homogeneous deposit of IgG in walls of small blood vessels and a weak linear band along the dermo-epidermal junction (×400) |
The differential diagnoses of pauci-cellular subepidermal blisters include bullous pemphigoid, epidermolysis bullosa acquisita, porphyria cutanea tarda and pseudoporphyria. Bullous pemphigoid clinically presents with pruritus and blisters on an erythematous base with a predilection for flexural regions. Scarring and milia formation are not generally seen and there is no mechanical fragility or photodistribution of lesions. Epidermolysis bullosa acquisita exhibits clinical features overlapping with pseudoporphyria. Direct immunofluorescence findings are useful in distinguishing bullous pemphigoid and epidermolysis bullosa acquisita from porphyria cutanea tarda/pseudoporphyria. In bullous pemphigoid and epidermolysis bullosa acquisita, direct immunofluorescence studies show a sharp linear band at the dermoepidermal junction while the vessel wall immune deposits characteristic of porphyria cutanea tarda/pseudoporphyria are absent. The distinction between porphyria cutanea tarda and pseudoporphyria is based on porphyrin levels which are raised in the former.
In our patient, skin lesions resolved within 3 weeks of drug withdrawal. Imatinib had to be reintroduced after 2 months due to worsening of the underlying hematological disorder. Her cutaneous symptoms recurred, but were milder because of photoprotection.
Pseudoporphyria is a disorder with clinical, histopathological and immunofluorescence features that are similar to porphyria cutanea tarda but without the biochemical porphyrin abnormalities. It presents as blistering and skin fragility on sun-exposed sites. Unlike porphyria cutanea tarda, hypertrichosis, hyperpigmentation, dystrophic calcification and sclerodermoid changes are seldom associated with pseudoporphyria.[2] The various causes of pseudoporphyria include ultraviolet A exposure, chronic renal failure/dialysis and a wide range of drugs, particularly nonsteroidal anti-inflammatory drugs, diuretics, antibiotics and retinoids.[2]
Imatinib has been implicated occasionally in causing both pseudoporphyria and porphyria cutanea tarda. We were able to find seven previously reported cases of imatinib-induced pseudoporphyria and three cases of imatinib precipitated/reactivated porphyria cutanea tarda in the English literature.[2],[3],[4] Two cases which were originally reported as skin fragility and blistering due to imatinib were subsequently categorized as pseudoporphyria in a review by Mahon et al.[3],[5],[6]
The pathogenesis of pseudoporphyria may differ depending on the causative factor; however, ultraviolet A exposure seems to be a common prerequisite. Various patho-mechanisms have been hypothesized in the causation of imatinib-induced pseudoporphyria. It has been suggested that imatinib, by inhibiting c-kit signaling in skin, might disrupt normal melanocyte biology leading to decreased photoprotection.[2],[3] A recent experimental study has proposed that direct photosensitization by imatinib or its metabolites is unlikely and that photosensitive disorders might be due to impaired melanogenesis or accumulation of endogenous porphyrins.[7]
Treatment of pseudoporphyria often includes discontinuation or reducing the dose of the offending drug or switching to a non-implicated drug and/or photoprotection. However, clinicians and patients have to reach a decision together, after weighing the adverse effects and therapeutic benefits of the implicated drug.
Considering the widespread use of imatinib in hematological malignancies and various other indications, clinicians must be aware that pseudoporphyria and porphyria cutanea tarda are possible adverse effects of the drug.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Pretel-Irazabal M, Tuneu-Valls A, Ormaechea-Pérez N. Adverse skin effects of imatinib, a tyrosine kinase inhibitor. Actas Dermosifiliogr 2014;105:655-62.
[Google Scholar]
|
| 2. |
Timmer-de Mik L, Kardaun SH, Kramer MH, Hayes DP, Bousema MT. Imatinib-induced pseudoporphyria. Clin Exp Dermatol 2009;34:705-7.
[Google Scholar]
|
| 3. |
Mahon C, Purvis D, Laughton S, Bradbeer P, Teague L. Imatinib mesylate-induced pseudoporphyria in two children. Pediatr Dermatol 2014;31:603-7.
[Google Scholar]
|
| 4. |
Au WY, Lee J. Imatinib mesylate (STI-571) and porphyria cutanea tarda in a Chinese patient. Haematologica 2005;90:ECR18.
[Google Scholar]
|
| 5. |
Verma SM, Murphy G. Skin fragility and blistering with imatinib mesylate. J Eur Acad Dermatol Venereol 2010;24:496-8.
[Google Scholar]
|
| 6. |
Reddy H, Horne HL, Maung Z. Skin fragility and blistering secondary to imatinib. Clin Exp Dermatol 2012;37:572-3.
[Google Scholar]
|
| 7. |
Nardi G, Lhiaubet-Vallet V, Miranda MA. Photosensitization by imatinib. A photochemical and photobiological study of the drug and its substructures. Chem Res Toxicol 2014;27:1990-5.
[Google Scholar]
|
Fulltext Views
3,942
PDF downloads
2,993





