Translate this page into:
In vitro antifungal susceptibility of Malassezia isolates from pityriasis versicolor lesions
2 Department of Biotechnology, Gauhati University, Guwahati, Assam, India
Correspondence Address:
Ajanta Sharma
Department of Microbiology, Gauhati Medical College, Gauhati - 781 032, Assam
India
| How to cite this article: Sharma A, Rabha D, Ahmed G. In vitro antifungal susceptibility of Malassezia isolates from pityriasis versicolor lesions. Indian J Dermatol Venereol Leprol 2017;83:249-251 |
Sir,
Pityriasis versicolor is the only human disease for which Malassezia has been fully established as a pathogen. The genus Malassezia includes 15 lipophilic species with the recent addition of one new species ”Malassezia arunalokei”. Traditionally, Malassezia furfur, Malassezia sympodialis, Malassezia globosa and Malassezia restricta have been considered the major pathogenic species implicated in dermatological disorders.[1],[2] Since Malassezia species are a part of the normal flora of skin, it is impossible to eradicate them permanently by topical and systemic antifungals resulting in relapses in predisposed individuals. Antifungal susceptibility testing is warranted for Malassezia yeasts, as they are implicated in both cutaneous and invasive infections in humans.
Because of the lipophilic nature, antifungal susceptibility testing of Malassezia yeasts is still a problem and hence, little work has been published on the in vitro susceptibilities of Malassezia to various antifungal agents. Various workers have evaluated the antifungal susceptibility of Malassezia employing modified Clinical Laboratory Standard Institute (CLSI) broth microdilution technique, using different culture media. These studies have reported significant variations in minimum inhibitory concentrations resulting in erroneous susceptibility classification. Hence, the present study aimed at the evaluation of in vitro susceptibility of Malassezia species to amphotericin B, ketoconazole, fluconazole, itraconazole and voriconazole by Clinical Laboratory Standard Institute (CLSI) protocol M27-A3 using modified Christensen's urea broth.[3],[4]
During the period 2012-2015, in the Department of Microbiology, Gauhati Medical college, Assam, Malassezia species were isolated from 290 patients with pityriasis versicolor and identified as M furfur (241), M. globosa (27), M. restricta (8), M. obtusa (7), M. sympodialis (5), M. slooffiae (1) and M. japonica (1) by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) of 26SrDNA region followed by sequencing.[5] Reference strains of Malassezia (M. furfur Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India, MTCC1374, M. globosa Centraalbureau Schimmelcultures-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands CBS7886, M. restricta CBS7877, M. japonica CBS9432, M. slooffiae CBS7956 and M. pachydermatis MTCC1369) and quality control strains C. albicans ATCC (American Type Culture Collection) 90028 and C. krusei (American Type Culture Collection) 6258 were tested as controls.
The concentration of the yeast suspensions (106 cfu/ml) were adjusted by spectrophotometer.[4] Stock suspensions of amphotericin B, ketoconazole, fluconazole, itraconazole and voriconazole (Sigma-Aldrich, USA), were prepared in dimethyl sulfoxide. The different drug concentrations varied between 0.125-64 μg/ml for fluconazole and between 0.0313-16 μg/ml for all other antifungals. Antifungal susceptibility testing was performed in 96-well microtiter plates and cultures were incubated at 32°C ± 2°C for 96 hours for M. globosa and M. restricta and 72 hours for other species.[3],[4] The final mean optical density obtained for each antifungal concentration was expressed as percentage of growth control. For azoles, the minimum inhibitory concentration endpoints of the antifungals were defined as the lowest drug concentrations that showed an optical density of ≤50% of that of the (drug-free) growth control. For amphotericin B, minimum inhibitory concentration endpoint was defined as the lowest concentration that completely inhibited growth.[4]
[Table - 1] summarizes the minimum inhibitory concentration (range, geometric mean & mode) and minimum inhibitory concentrations where 50% and 90% of the isolates were inhibited (MIC50 and MIC90) obtained for the antifungal drugs. The minimum inhibitory concentration of ketoconazole, itraconazole and voriconazole was 1 μg/ml for 90% of the M. furfur and M. globosa isolates; however, the minimum inhibitory concentration of amphotericin B for M. furfur was higher than that for M. globosa (1 μg/ml versus 0.5 μg/ml). Fluconazole minimum inhibitory concentrations were higher than other azoles and ranged from ≤0.12 to >64 μg/ml for M. furfur, ≤0.12 to 8 μg/ml for M. globosa and M. restricta. M. globosa showed higher minimum inhibitory concentration ranges to all the azoles. The geometric mean and mode for all drugs tested were higher for M. furfur, M. globosa and M. restricta especially for fluconazole, itraconazole and voriconazole.
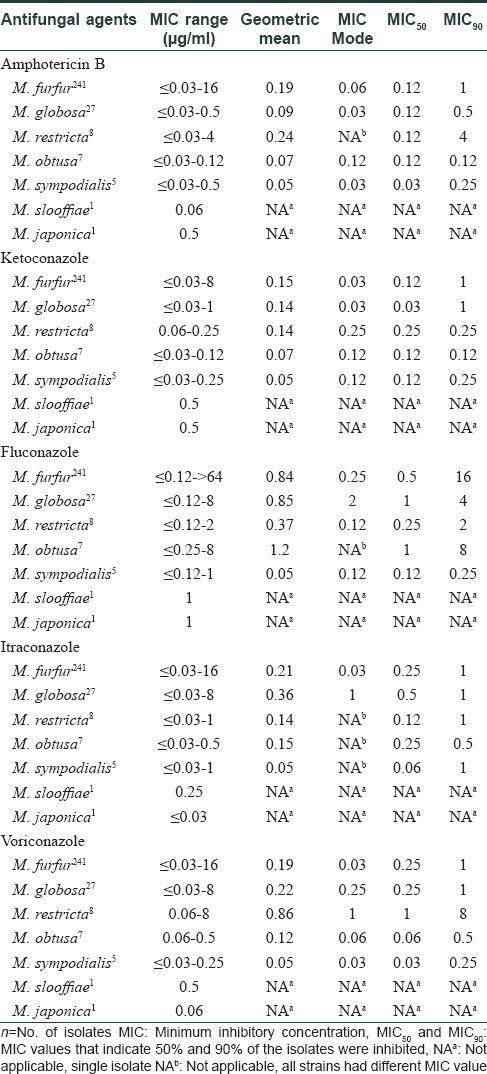
In this study, the Malassezia species could be divided into two groups. M. sympodialis, M. obtusa, M. slooffiae and M. japonica were more susceptible to antifungals while M. furfur, M. globosa and M. restricta comprised the less susceptible group. Every result obtained using this method demonstrated good reproducibility. The overall data shows that, the non-applicability of Clinical Laboratory Standard Institute M27-A3 protocol for Malassezia species has resulted in variations in methodologies for minimum inhibitory concentration determination with limited inter laboratory agreement. The original Clinical Laboratory Standard Institute protocol has been modified by using a more suitable growth medium, increasing the inoculum size to counteract the slower growth of Malassezia, increasing incubation time and altering the definition of the minimum inhibitory concentration end point.
In conclusion, modified Christensen's urea broth may be used for antifungal susceptibility testing of Malassezia with optimum testing conditions such as standardization of inocula, incubation temperature and time.
Acknowledgement
I gratefully acknowledge the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, for their financial support in this work under Research & Development project grant vide sanction number BT/PR3633/MED/29/330/2011 dated 26/03/2011. I also express my sincere gratitude to Dr. Arunaloke Chakrabarti, Professor and Head, Dr. M.R. Shivaprakash, Additional Professor, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh for providing some of the reference strains.
Financial support and sponsorship
Department of Biotechnology (DBT), Ministry of Science and Technology, Govt. of India, New Delhi.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev 2012;25:106–41.
[Google Scholar]
|
| 2. |
Honnavar P, Prasad GS, Ghosh A, Dogra S, Handa S, Rudramurthy SM. Malassezia arunalokei sp. nov., a novel yeast species isolated from seborrhoeic dermatitis patients and healthy individuals from India. J Clin Microbiol 2016;54:1826-34.
[Google Scholar]
|
| 3. |
CLSI. Reference Method for Broth dilution antifungal susceptibility testing of yeasts; approved standard, CLSI document M27-A3. -3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
[Google Scholar]
|
| 4. |
Rincón M, Cepero de García C, Espinel-Ingroff A. A Modified Christensen's Urea and CLSI Broth Microdilution Method for Testing Susceptibilities of Six Malassezia Species to Voriconazole, Itraconazole, and Ketoconazole. J Clin Microbiol 2006;44:3429-31.
[Google Scholar]
|
| 5. |
Mirhendi H, Makimura K, Zomorodian K, Yamada T, Sugita T, Yamaguchi H. A simple PCR-RFLP method for identification and differentiation of 11 Malassezia species. J Microbiol Methods2005;61:281-4.
[Google Scholar]
|
Fulltext Views
3,094
PDF downloads
1,851





