Translate this page into:
Increased serum levels of interleukin-6 in erythema nodosum leprosum suggest its use as a biomarker
Corresponding author:Dr. Fátima Regina Vilani Moreno, Lauro de Souza Lima Institute, Rod. Comte. João Ribeiro de Barros, Km 225/226, 17034-971, Bauru, SP, Brazil. frvmoreno@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vilani-Moreno FR, Brito-de-Souza VN, Silva SUR, Barbosa AS, Sartori BG, Campanelli AP, et al. Increased serum levels of interleukin-6 in erythema nodosum leprosum suggest its use as a biomarker. Indian J Dermatol Venereol Leprol 2021;87:190-8.
Abstract
Background:
Erythema nodosum leprosum (ENL) is a frequent complication of multibacillary leprosy that can result in significant morbidity, including peripheral nerve damage and physical disability. The identification of possible serum markers could be a valuable tool for the early detection of ENL.
Aims:
The purpose of this study was to evaluate selected serum mediators involved in the innate and adaptive immune responses to identify possible immunomarkers for ENL.
Methods:
The levels of interleukin-2, interleukin-4, interleukin-6, interleukin-10, interleukin-17, interferon-γ, tumor necrosis factor, nitric oxide and anti-phenolic glycolipid-I antibodies were measured in the sera of leprosy patients with ENL [at the beginning of reaction (M0) and 1 month later (M1)], and then compared with the levels of the same markers in patients with untreated multibacillary leprosy without ENL (controls with leprosy: CTRL) and healthy individuals (healthy controls: CTRH).
Results:
Significantly higher levels of serum interleukin-6 were observed in M0 than in CTRL. In addition, pairwise comparisons showed higher levels of interleukin-6 in M0 compared to M1. Levels of tumor necrosis factor were higher in M0 than in CTRL, with no significant difference between M0 and M1. There were no differences in the levels of interleukin-2, interleukin-4, interleukin-10, interleukin-17 or interferon-γ between groups. The CTRL group had higher levels of nitric oxide compared to M0 and M1. High levels of anti-phenolic glycolipid-I were observed in M0, M1 and CTRL than in CTRH.
Limitations:
Three patients were not assessed at M1, decreasing the number of evaluated patients from 14 to 11.
Conclusion:
High-serum levels of interleukin-6 were observed during ENL, primarily in patients with more severe reactions; levels decreased after specific therapy, suggesting a role for this cytokine in pathogenesis and its utility as an ENL biomarker. Further studies should explore whether interleukin-6 could also be used as a predictive marker for ENL or as a specific target for its treatment.
Keywords
Biomarker
cytokines
erythema nodosum
interleukin 6
leprosy
Plain Language Summary
Leprosy is an infectious disease that affects nerves and skin. Some leprosy patients can suffer a kind of systemic inflammation known as erythema nodosum leprosum (ENL), that is characterized by the presence of painful nodules in the skin, fever, malaise and pain in the muscles and bones and may cause damage in nerves. Thus, the authors conducted a study in order to find some protein in the blood that could be used to help in early diagnosis of the condition. The study was carried out at Lauro de Souza Lima Institute, in Bauru, Brazil. It included leprosy patients with and without ENL and healthy individuals. The presence of some proteins that control the immune response, called cytokines, was assessed in the blood and the authors found that one protein, called interleukin-6 (IL-6), was increased only in the blood of patients with ENL. The authors suggested that IL-6 could be used as a marker for ENL.
Introduction
Erythema nodosum leprosum (ENL), or type 2 reaction, is an acute inflammatory complication that overlaps the chronic and insidious evolution of multibacillary leprosy and represents an important cause of morbidity and physical disability. ENL occurs mainly during or after treatment with multidrug therapy, but there are also cases in which it is the first manifestation, even before a diagnosis of leprosy.1
ENL affects patients with lepromatous leprosy and borderline lepromatous leprosy, known as multibacillary patients. It is characterized by the sudden appearance of papules, nodules, and painful plaques in the skin, which can progress to pustules and blisters with subsequent ulceration and necrosis in the most severe cases. Systemic manifestations can occur, including fever, malaise, generalized lymphadenopathy, pain in the muscles and bones, arthritis, iritis, iridocyclitis, orchitis, epididymitis, glomerulonephritis and neuropathy. Edema of hands, feet and face has also been observed.1
Episodes of ENL may either regress rapidly or persist for years, depending on the inflammatory response of individual patients. Thus, early diagnosis and appropriate management are essential to minimize clinical manifestations and alleviate patient suffering thereby providing a better quality of life.
The histopathology of acute lesions of ENL shows neutrophilic infiltration overlapping the chronic lepromatous pattern often associated with vasculitis, as well as the presence of fragmented bacilli.2 The pathogenesis of ENL seems to be related to a predominantly Th2 cytokine profile and the presence of immune complexes.3 Increased expression of mRNAs encoding interleukin-6, interleukin-8 and interleukin-10 and sustained expression of mRNAs encoding interleukin-4 and interleukin-5 have also been reported.2,4 These cytokines are related to neutrophil chemotaxis, antibody production and reduced cell-mediated immune responses.
In contrast, other studies have demonstrated a role for Th1 responses in ENL through the expression of mRNAs encoding interferon-γ, tumor necrosis factor and interleukin-12.3,5,6 More recent studies have also indicated the involvement of Th17 cells with elevated levels of interleukin-17 in culture supernatants and skin lesions;7-9 however, no significant change in serum levels of interleukin-17 was observed in ENL.10-12
Given the importance of finding clinical and laboratory markers that recognize both ENL patients and those at increased risk of ENL development, the objective of our study was to evaluate serum mediators of innate and adaptive immune responses, including both pro and anti-inflammatory cytokines, to identify laboratory markers for ENL.
Methods
Subjects
Fourteen leprosy patients classified according to Ridley and Jopling (seven lepromatous leprosy and seven borderline lepromatous leprosy, twelve males and two females, mean age 56 years), with confirmed diagnoses of ENL by clinical and histopathological evaluations, were enrolled before initiating specific treatment for ENL.13 Among these patients, eleven were evaluated again 1 month later (after recovery). In addition, sixteen patients with multibacillary leprosy but not treated with multidrug therapy and without ENL reactions (controls with leprosy: CTRL, seven lepromatous leprosy and nine borderline lepromatous leprosy, twelve males and four females, mean age 50 years) and fifteen healthy individuals (healthy controls: CTRH, eleven males and four females, mean age 51 years) were also recruited. The clinical status of ENL patients was evaluated according to Walker et al.14 Briefly, mild reaction was defined as 1–10 ENL skin lesions with a little pain on palpation, no fever and absence of systemic signs and symptoms; moderate as 11–20 ENL skin lesions that were painful on palpation, fever between 37.6 and 38.5°C, edema of hands, face or feet at two sites, with pain or tenderness in lymph-node chains; and severe as 21 or more painful ENL skin lesions, ulcerated lesions involving a large area of the tegument, edema of hands, face and feet, high fever (>38.5°C), generalized involvement of lymph node chains and systemic signs and symptoms. Clinical characteristics of the ENL patients are shown in Table 1. Patients and controls were recruited at Lauro de Souza Lima Institute, Bauru, and in the Family Health Program, Rondonópolis, Brazil.
| Patient/clinical form | Sex | Age (years) | ENL ab initio | ENL x MDT | ENL post-MDT | ENL severity | ENL post-M1* |
|---|---|---|---|---|---|---|---|
| 1/BL | Male | 57 | X | Mild | X | ||
| 2/LL | Male | 40 | X | Severe | X | ||
| 3/BL | Male | 64 | X | Moderate | X | ||
| 4/BL | Male | 66 | X | Severe | X | ||
| 5/LL | Male | 22 | X | Severe | X | ||
| 6/BL | Male | 74 | X | Moderate | X | ||
| 7/BL | Male | 56 | X | Moderate | |||
| 8/BL | Male | 56 | X | Severe | • | ||
| 9/BL | Female | 51 | X | Moderate | X | ||
| 10/LL | Male | 62 | X | Mild | X | ||
| 11/LL | Male | 50 | X | Severe | |||
| 12/LL | Female | 76 | X | Moderate | X | ||
| 13/LL | Male | 65 | X | Moderate | X | ||
| 14/LL | Male | 50 | X | Moderate | X |
Ethical statement
The study protocol was approved by the ethics committee of Lauro de Souza Lima Institute (number 1,824,819). Written informed consent was obtained from all participants after they were provided with a complete description of the study.
Blood collection
Peripheral blood (10 mL) was collected from each participant to obtain serum, which was aliquoted and stored at −80°C. For patients with ENL, blood was collected both at the beginning of the reactional episode, before of the specific treatment for the reaction (M0) and 1 month later (M1).
Quantification of cytokines
Serum levels of interleukin-2, interleukin-4, interleukin-6, interleukin-10, interleukin-17, interferon-γ and tumor necrosis factor were measured by flow cytometry using Th1/Th2/Th17 cytometric beads array kits according to the manufacturer’s instructions (BD Biosciences, San Jose, CA, USA). Briefly, 50 μL of cytokine capture beads were incubated with 50 μL of serum and 50 μL of the detection antibody conjugated with fluorochrome phycoerythrin for 3 h at room temperature in the dark. The beads were washed by centrifugation and the samples were analyzed with a FACSCalibur flow cytometer (BD). Data were analyzed against a standard curve using FCAP Array software (BD). Serum levels of cytokines below the detection limit were expressed as 0 pg/mL.
Production of nitric oxide
Detection of serum nitric oxide was based on the measurement of nitrite (NO2−) by the colorimetric Griess reaction using n-(1-naphthyl) ethylenediamine and sulfanilamide. 15 After incubation for 10 min at room temperature, the reaction was read in an enzyme-linked immunosorbent assay microplate reader at 540 nm against a blank solution consisting of Griess reagent. The results (expressed as μmol) were generated by comparing the optical density of the reactions to a standard curve prepared for each assay.
Quantification of anti-phenolic glycolipid-I antibodies
Detection of IgM anti-phenolic glycolipid-I antibodies was performed using enzyme-linked immunosorbent assay as described by Brett et al. 16 Briefly, 96-well plates were coated with ND-O-BSA (natural disaccharide linked to bovine serum albumin, via an octyl) semi-synthetic antigen at 20 ng/mL (kindly provided by Dr. John Spencer, Colorado State University, USA) and blocked with 1% bovine serum albumin in phosphate-buffered saline (pH 7.4). Sera were diluted 1:500 in phosphate-buffered saline containing 0.1% Tween 20 and 10% normal goat serum (Gibco, Grand Island, NY, USA) and tested in duplicate. After incubation and washing, peroxidase-conjugated rabbit anti-human IgM polyclonal antibody (Dako Denmark A/S, Glostrup, Denmark) was added at a 1:10,000 dilution in phosphate-buffered saline containing 0.1% Tween 20 and 10% normal goat serum. After another incubation and washing, substrate [1% tetramethylbenzidine in dimethyl sulfoxide and 3.5 mM hydrogen peroxide in 0.1 M citrate buffer/acetate buffer, pH 6.0] was added and the reaction was stopped by the addition of 2.0 N H2SO4. The enzymatic activity was read in an enzyme-linked immunosorbent assay microplate reader using a 450-nm filter. Sera with optical density ≥ 0.2 were considered positive.17
Statistical analyses
Kruskal–Wallis one-way ANOVA followed by Dunn’s multiple comparisons test was used to verify differences between groups. The Wilcoxon test was used for pairwise comparisons between M0 and M1. Data were analyzed using GraphPad Prism 5 software (GraphPad Inc., USA) and the differences were considered significant at P < 0.05.
Results
ENL patients had higher serum levels of interleukin-6 at M0 [median: 195.21 pg/mL, interquartile range: 28.18– 440.57 pg/mL] compared to CTRL (0.37 pg/mL, interquartile range: 0.00–3.24 pg/mL) (P < 0.001) [Figure 1a]. Furthermore, the analyses of paired date (n = 11) showed that the production of interleukin-6 by ENL patients at M0 (117.99 pg/mL, interquartile range: 25.17–389.86 pg/mL) was higher than at M1 (9.18 pg/mL, interquartile range: 3.58–25.25 pg/mL) (P = 0.027) [Figure 1b]. Moreover, the level of interleukin-6 was higher in patients with severe ENL (median: 384.28 pg/mL) relative to those presenting with moderate (median: 195.21 pg/mL) and mild ENL (median: 58.32 pg/mL) [Table 2].
| Patient/clinical form | ENL severity | IL-6/M0 | IL-6/M1 | TNF/M0 | TNF/M1 |
|---|---|---|---|---|---|
| 1/BL | Mild | 0.43 | 61.70 | 0 | 0 |
| 10/LL | Mild | 116.21 | 90.64 | 50.37 | 66.97 |
| Median | Mild | 58.32 | 76.17 | 25.19 | 33.49 |
| 3/BL | Moderate | 357.73 | 11.64 | 504.41 | 95.28 |
| 6/BL | Moderate | 470.41 | 3.04 | 7.16 | 74.48 |
| 7/BL | Moderate | 195.21 | Not done | 0.62 | Not done |
| 9/BL | Moderate | 24.17 | 27.42 | 2.44 | 65.14 |
| 12/LL | Moderate | 717.11 | 18.75 | 67.39 | 0 |
| 13/LL | Moderate | 119.76 | 5.20 | 0 | 0 |
| 14/LL | Moderate | 6.89 | 0 | 0 | 2.56 |
| Median | Moderate | 195.21 | 8.42 | 2.44 | 33.85 |
| 2/LL | Severe | 28.18 | 6.72 | 18.92 | 31.64 |
| 4/BL | Severe | 367.99 | Not done (recurrent ENL) | 99.33 | Not done (recurrent ENL) |
| 5/LL | Severe | 638.50 | Not done (recurrent ENL) | 1.40 | Not done (recurrent ENL) |
| 8/BL | Severe | Not done | Death | 0 | Death |
| 11/BL | Severe | 400.57 | 0 | 16.73 | 0 |
| Median | Severe | 384.28 | 3.36 | 16.73 | 15.82 |
IL: Interleukin, TNF: Tumor necrosis factor, ENL: Erythema nodosum leprosum, BL: Borderline lepromatous, LL: Lepromatous leprosy
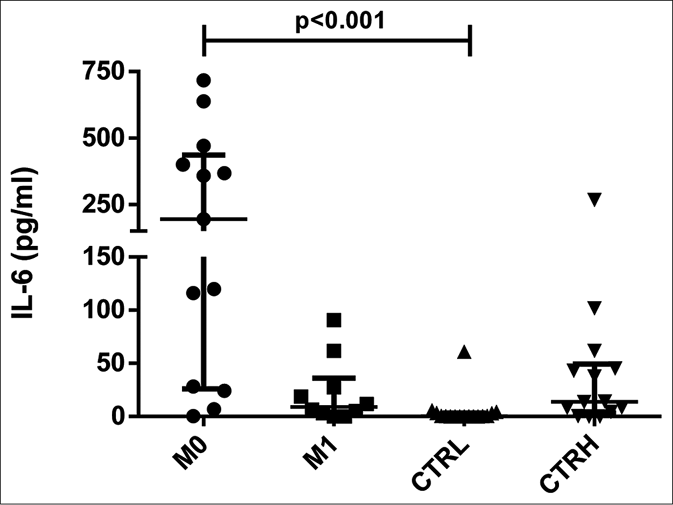
- Serum levels of interleukin-6 in erythema nodosum leprosum patients at the moment of reaction (M0) and 1 month postreaction (M1), leprosy patients without reaction (CTRL) and healthy individuals (CTRH). Bars represent median and interquartile range

- Pairwise comparisons of serum levels of interleukin-6 in erythema nodosum leprosum patients at the moment of reaction (M0) and 1 month postreaction (M1)
The serum levels of tumor necrosis factor were higher in both ENL patients at M0 (4.80 pg/mL, interquartile range: 0.16– 42.51 pg/mL) and CTRH (78.15 pg/mL, interquartile range: 17.18–144.25 pg/mL) than in CTRL (0.00, interquartile range: 0.00–0.00 pg/mL) (P = 0.041 and P < 0.001, respectively) [Figure 2a]. When the results were analyzed with paired data for ENL patients, there was no significant difference between M0 and M1 (11.95 pg/mL, interquartile range: 0.61–42.51 pg/mL and 17.10 pg/mL, interquartile range: 0.00–66.51 pg/mL, respectively) [Figure 2b]. Tumor necrosis factor levels in patients with mild ENL (median: 25.19 pg/ mL) were higher than in patients with severe (median: 16.73 pg/mL) and moderate ENL (median: 2.44 pg/mL) [Table 2].
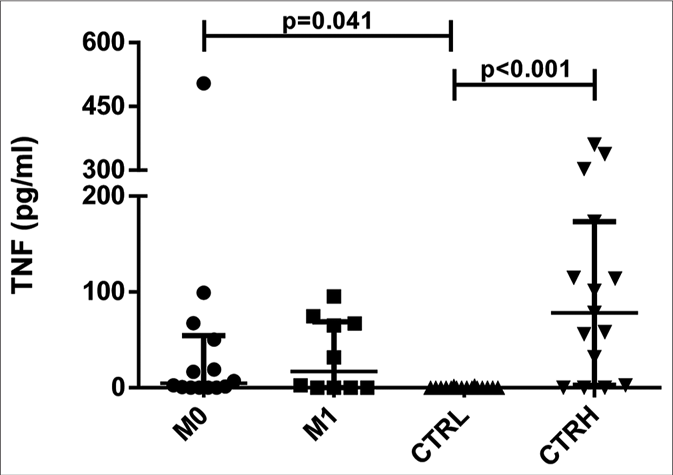
- Serum levels of tumor necrosis factor in erythema nodosum leprosum patients at the moment of reaction (M0) and 1 month postreaction (M1), leprosy patients without reaction (CTRL) and healthy individuals (CTRH). Bars represent median and interquartile range
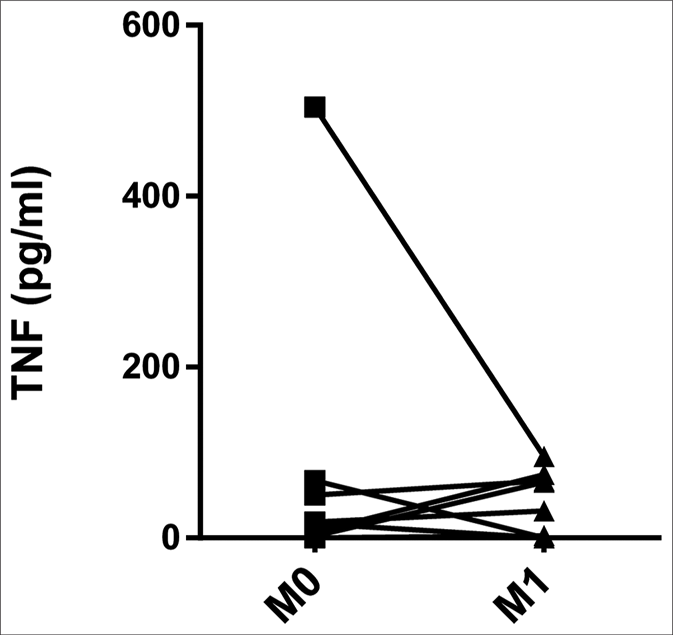
- Pairwise comparisons of serum levels of tumor necrosis factor in erythema nodosum leprosum patients at the moment of reaction (M0) and 1 month postreaction (M1)
There were no significant differences in the serum levels of interleukin-2, interleukin-4, interleukin-10, interleukin-17 and interferon-γ between M0 and M1, between M0 and CTRL, M0 and CTRH, M1 and CTRL, M1 and CTRH, and between CTRL and CTRH [Table 3]. Moreover, there was also no difference when the results were analyzed with paired data. It should be noted that serum levels of interleukin-2 and interleukin-4 at M0 and M1 were below the detection limits [Table 3].
| Patients/controls | IL-2 | IL-4 | IL-10 | IL-17 | IFN-γ |
|---|---|---|---|---|---|
| M0 | 0.00 0.00-0.00 |
0.00 0.00-0.00 |
0.00 0.00-3.36 |
0.00 0.00-62.32 |
0.00 0.00-4.85 |
| M1 | 0.00 0.00-0.00 |
0.00 0.00-0.00 |
0.00 0.00-4.04 |
0.00 0.00-23.72 |
0.00 0.00-0.00 |
| CTRL | 0.00 0.00-0.25 |
0.00 0.00-0.00 |
0.00 0.00-0.00 |
0.00 0.00-32.16 |
0.00 0.00-1.79 |
| CTRH | 0.00 0.00-57.15 |
0.00 0.00-39.43 |
0.00 0.00-39.69 |
0.00 0.00-0.86 |
0.00 0.00-0.64 |
IL: Interleukin, IFN: Interferon
The CTRL group had higher serum levels of nitric oxide (64.42 μmol, interquartile range: 51.97–73.50 μmol) than did M0 (32.76 μmol, interquartile range: 22.68–42.51 μmol) and M1 (32.20 μmol, interquartile range: 13.54–38.32 μmol) (P = 0.010 and P = 0.002, respectively) [Figure 3a]. The pairwise comparisons showed no significant differences between M0 (33.50 μmol, interquartile range: 22.71–49.58 μmol) and M1 (32.20 μmol, interquartile range: 13.54–38.32 μmol) [Figure 3b].
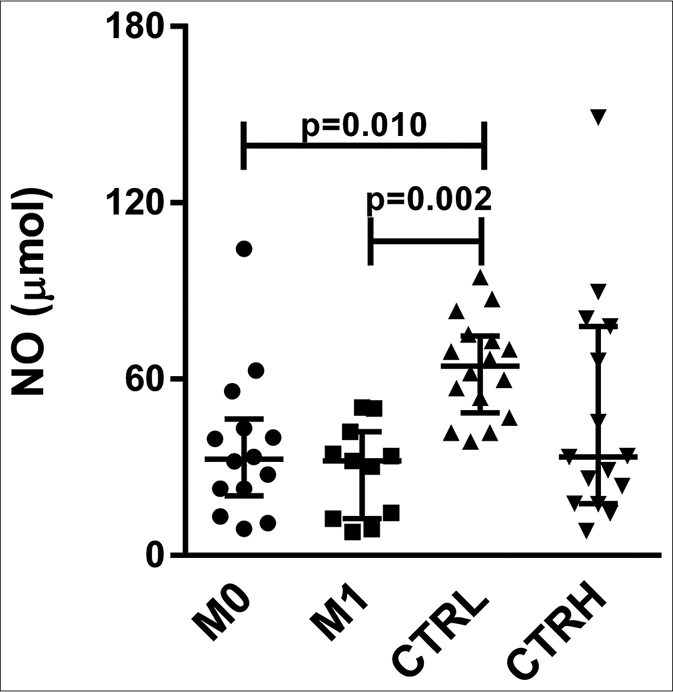
- Serum levels of nitric oxide in erythema nodosum leprosum patients at the moment of reaction (M0) and 1 month postreaction (M1), leprosy patients without reaction (CTRL) and healthy individuals (CTRH). Bars represent median and interquartile range
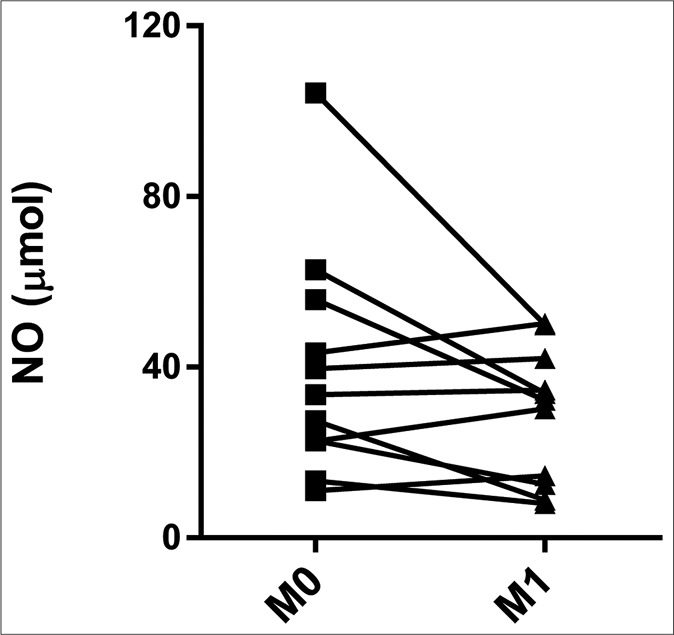
- Pairwise comparisons between M0 and M1
The levels of anti-phenolic glycolipid-I antibodies were higher in ENL patients at M0 (optical density: 0.274, interquartile range: 0.154–0.696), M1 (optical density: 0.319, interquartile range: 0.110–0.774), and CTRL (optical density: 0.393, interquartile range: 0.227–0.922) compared to CTRH (optical density: 0.034, interquartile range: 0.020–0.060) (P < 0.001, P = 0.001 and P < 0.001, respectively) [Figure 4a]. The pairwise comparisons revealed no difference between M0 (optical density: 0.380, interquartile range: 0.124– 0.745) and M1 (0.319, interquartile range: 0.110–0.774) for anti-phenolic glycolipid-I antibodies [Figure 4b].
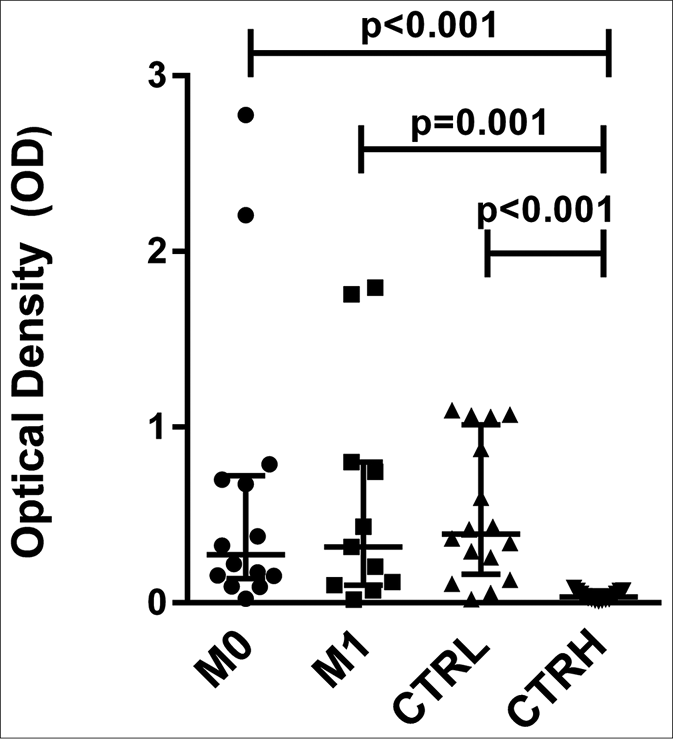
- Serum levels of antiphenolic glycolipid-I antibodies from erythema nodosum leprosum patients at the moment of reaction (M0) and 1 month postreaction (M1), leprosy patients without reaction (CTRL) and healthy individuals (CTRH). Bars represent median and interquartile range
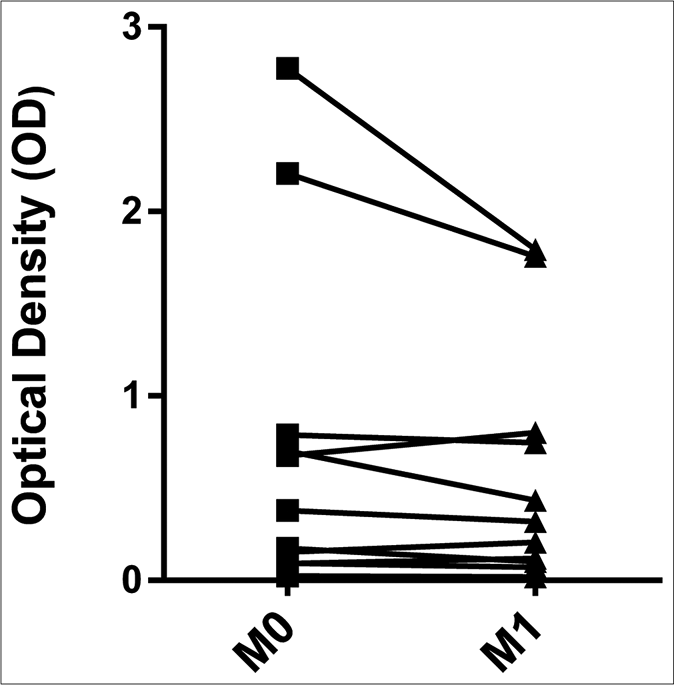
- Pairwise comparisons between M0 and M1
Discussion
We have evaluated serum levels of interleukin-2, interleukin-4, interleukin-6, interleukin-10, interleukin-17, interferon-γ and tumor necrosis factor using Th1/Th2/Th17 cytometric beads array kits by flow cytometry, which has been widely used18-20 to measure cytokines in small volumes of serum without losing sensitivity, accuracy or reproducibility. 21 Our results showed low levels of interferon-γ, interleukin-10 and interleukin-17 without a significant difference between M0 and M1, or between these time points and the control groups. In addition, interleukin-2 and interleukin-4 levels were below detection limits at M0 and M1. Therefore, these serum cytokines do not appear to be relevant to ENL, in contrast to the elevated levels of interleukin-6 and tumor necrosis factor during ENL episodes observed in this study.
Of special interest, serum levels of interleukin-6 were higher in ENL at M0 than in M1 and the control groups (leprosy patients without reaction and healthy individuals). Results from pairwise comparisons revealed a significant difference in interleukin-6 levels between M0 and M1, suggesting that this cytokine has a role in ENL episodes and could be used as a marker for ENL in multibacillary leprosy patients. To date, there has been some research on laboratory parameters in ENL reactions; however, the results have been contradictory and with limited applicability.17,22,23
Studies evaluating serum levels of interleukin-6 in ENL have shown discordant results, with some reporting elevated levels,24-26 similar to our findings, while others reported no association between interleukin-6 and ENL.23,27 More recent studies have revealed the association of single-nucleotide polymorphisms in the IL6 gene with ENL, with corresponding elevated serum interleukin-6 levels, consistent with our findings.28,29 Considering the high levels of interleukin-6 during ENL reported in the literature24-26,28,29 and its decrease after the remission of the reaction demonstrated by Villahermosa et al.30 and our results, it is reasonable to propose an important role for interleukin-6 in ENL. To further support this hypothesis, we observed an association between interleukin-6 and the severity of ENL reaction, with higher serum levels of this cytokine in patients with severe ENL than in those with moderate or mild reaction.
Interleukin-6 is a proinflammatory cytokine that promotes the maturation and activation of neutrophils and stimulates the production of antibodies. Neutrophilic infiltration is involved in the initial phase of ENL reactions and decreases progressively over 9 days, representing the transition from the acute phase to the regressive phase of the reaction.3,31 Furthermore, since interleukin-6 is synthesized in response to infections or tissue damage and stimulates the production of acute-phase proteins in hepatocytes, it is possible that the decreased serum levels of interleukin-6 observed at M1 are related to the regression of the inflammatory process.
Consistent with previous studies, our results revealed elevated serum levels of tumor necrosis factor in ENL patients at M0 in comparison to CTRL.23,27,32-34 This finding, together with our previous observation of elevated tumor necrosis factor mRNA expression in ENL, suggests that this cytokine could also be used as an immunomarker for ENL.35 However, there was no significant difference in serum tumor necrosis factor levels nor tumor necrosis factor mRNA expression between M0 and M1, although the values of this cytokine were slightly lower in M1.35 Tumor necrosis factor levels have been shown to decrease in response to thalidomide, an effective drug in the treatment of ENL.27,36,37 In this study, no patient was using thalidomide at M0, but six patients had used this drug between M0 and M1 and two of them had recurrent ENL, so they were excluded from the assessment at M1. Our results are in agreement with a study conducted by Haslett et al., in which no difference was observed in tumor necrosis factor serum levels in ENL patients between the onset of the reaction and 28 days postreaction.38
In the present study, we also measured serum nitric oxide levels, since this metabolite could signal the participation of a cellular immune response (Th1), which has been suggested to be involved in ENL.3,5,6 Our results showed higher levels of nitric oxide in CTRL than in M0, suggesting that nitric oxide does not actively participate in the reaction. We previously quantified nitric oxide in the supernatants of peripheral blood mononuclear cells culture from ENL patients and did not find a significant difference relative to healthy controls, consistent with findings from the present study. 35
The serum levels of anti-phenolic glycolipid-I antibodies, an indicator of humoral immune response, were elevated in M0, M1 and CTRL, with no significant difference between reactional and non-reactional groups as observed by others.17,39,40 However, conflicting observations have also been reported, showing lower levels in ENL, possibly due to the formation and deposition of immune complexes in tissues based on the pathophysiology of this reaction.41,42
Limitations
One limitation of this study was that two patients had recurrent ENL and one died; therefore, they were excluded from the assessment at M1 and the number of ENL patients was reduced from 14 to 11 at M1.
Conclusion
The follow-up of 13 ENL patients for two years showed that 11 patients had new episodes of ENL, reinforcing the importance of identifying biomarkers that may indicate early development of reaction.
Our results have shown that ENL patients have low serum levels of interferon-γ, interleukin-10 and interleukin-17 and high levels of interleukin-6 and tumor necrosis factor. More importantly, interleukin-6 was elevated only during ENL episodes (M0), reinforcing its role in the pathogenesis of this reaction and suggesting its utility as an immunomarker for ENL and a valuable indicator for the risk of ENL. Further studies should be conducted to address this possibility. An improved understanding of the actions of the cytokines and mediators during ENL can help develop new strategies for both treatment and prevention to avoid or reduce nerve damage, which severely impairs the quality of life of leprosy patients.
Acknowledgements
We thank Dr. John Spencer of Department of Microbiology, Immunology and Pathology, Colorado State University, USA, for providing the phenolic glycolipid-I antigen.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Paulista Foundation against Hansen's disease.
Conflicts of interest
There are no conflicts of interest.
References
- Recognizing and managing the immunologic reactions in leprosy. J Am Acad Dermatol. 2014;71:795-803.
- [CrossRef] [PubMed] [Google Scholar]
- The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-81.
- [CrossRef] [PubMed] [Google Scholar]
- Towards understanding the pathology of erythema nodosum leprosum. Trans R Soc Trop Med Hyg. 2008;102:329-37.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470-5.
- [Google Scholar]
- Lepromatous leprosy patients show T helper 1 like cytokine profile with differencial expression of interleukin 10 during type 1 and 2 reactions. Immunology. 1998;95:529-36.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine mRNA expression in leprosy: A possible role for interferon gamma and interleukin 12 in reactions (RR and ENL) Scand J Immunol. 1999;50:541-9.
- [CrossRef] [PubMed] [Google Scholar]
- Lessons of leprosy: The emergence of TH17 cytokines during type II reactions (ENL) is teaching us about T cell plasticity. J Drugs Dermatol. 2012;11:626-30.
- [Google Scholar]
- Leprosy reactions show increased Th17 cell activity and reduced FOXP3+ Tregs with concomitant decrease in TGF β and increase in IL 6. PLoS Negl Trop Dis. 2016;10:e0004592.
- [CrossRef] [PubMed] [Google Scholar]
- In situ T regulatory cells and Th17 cytokines in paired samples of leprosy type 1 and type 2 reactions. PLoS One. 2018;13:e0196853.
- [CrossRef] [PubMed] [Google Scholar]
- Increased serum circulatory levels of interleukin 17F in type 1 reactions of leprosy. J Clin Immunol. 2012;32:1415-20.
- [CrossRef] [PubMed] [Google Scholar]
- Serum Th17 cytokines in leprosy: Correlation with circulating CD4(+) CD25 (high) FoxP3 (+) T regs cells, as well as down regulatory cytokines. Arch Dermatol Res. 2014;306:793-801.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous analysis of multiple T helper subsets in leprosy reveals distinct patterns of Th1, Th2, Th17 and Tregs markers expression in clinical forms and reactional events. Med Microbiol Immunol. 2017;206:429-39.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of leprosy according to immunity. A five group system. Int J Lepr Other Mycobact Dis. 1966;34:255-73.
- [Google Scholar]
- The development of a severity scale for Erythema Nodosum Leprosum-The ENLIST ENL severity scale. Lepr Rev. 2016;87:333-46.
- [CrossRef] [Google Scholar]
- Use of synthetic glycoconjugates containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin Exp Immunol. 1986;64:476-83.
- [Google Scholar]
- Utility of measuring serum levels of anti PGL I antibody, neopterin and C reactive protein in monitoring leprosy patients during multidrug treatment and reactions. Trop Med Int Health. 2007;12:1440-58.
- [CrossRef] [PubMed] [Google Scholar]
- The imbalance between Tregs, Th17 cells and inflammatory cytokines among renal transplant recipients. BMC Immunol. 2015;16:56.
- [CrossRef] [PubMed] [Google Scholar]
- Association of TNF α(308(GG)), IL 10( 819(TT)), IL 10( 1082(GG)) and IL 1R1(+1970(CC)) genotypes with the susceptibility and progression of leprosy in North Indian population. Cytokine. 2015;73:61-5.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokines as biomarkers to monitoring the impact of multidrug therapy in immune response of leprosy patients. Cytokine. 2017;97:42-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine measurement using cytometric bead arrays. Methods Mol Biol. 2012;845:425-34.
- [CrossRef] [PubMed] [Google Scholar]
- Serum neopterin as a marker for reactional states in leprosy. FEMS Immunol Med Microbiol. 1999;24:405-9.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of interferon gamma, tumour necrosis factor alpha, soluble interleukin 6R and soluble cell activation markers for monitoring response to treatment of leprosy reactions. Clin Exp Immunol. 2007;150:210-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pentoxifylline decreases in vivo and in vitro tumour necrosis factor alpha (TNF alpha) production in lepromatous leprosy patients with erythema nodosum leprosum (ENL) Clin Exp Immunol. 1998;111:300-8.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating cytokine profiles in leprosy patients. Lepr Rev. 2007;78:223-30.
- [CrossRef] [PubMed] [Google Scholar]
- Potential plasma markers of Type 1 and Type 2 leprosy reactions: A preliminary report. BMC Infect Dis. 2009;9:75.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J Infect Dis. 1993;168:408-14.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic and immunological evidence implicates interleukin 6 as a susceptibility gene for leprosy type 2 reaction. J Infect Dis. 2012;205:1417-24.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic polymorphisms of the IL6 and NOD2 genes are risk factors for inflammatory reactions in leprosy. PLoS Negl Trop Dis. 2017;11:e0005754.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, double blind, double dummy, controlled dose comparison of thalidomide for treatment of erythema nodosum leprosum. Am J Trop Med Hyg. 2005;72:518-26.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of tumour necrosis factor alpha and interleukin 1 beta during leprosy reactional states. Clin Exp Immunol. 1991;84:103-8.
- [CrossRef] [Google Scholar]
- Correlation between TNF production, increase of plasma C reactive protein level and suppression of T lymphocyte response to concanavalin A during erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1993;61:218-26.
- [Google Scholar]
- Serum cytokine profile in leprosy and its correlation with clinico histopathological profile. Lepr Rev. 2011;82:371-82.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the imune response in erythema nodosum leprosum: Quantification and expression of cytokines and analyses of the reactive oxygen and nitrogen intermediates. 19th International Leprosy Congress.
- Anti inflammatory drugs block cytokine mRNA accumulation in the skin and improve the clinical condition of reactional leprosy patients. J Invest Dermatol. 2000;115:935-41.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of pentoxifylline, thalidomide and prednisone in the treatment of ENL. Int J Lepr Other Mycobact Dis. 1998;66:61-5.
- [Google Scholar]
- Effective treatment of erythema nodosum leprosum with thalidomide is associated with immune stimulation. J Infect Dis. 2005;192:2045-53.
- [CrossRef] [PubMed] [Google Scholar]
- Erythema nodosum leprosum case series report: Clinical profile, immunological basis and treatment implemented in health services. Rev Soc Bras Med Trop. 2004;37:384-90.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of anti PGL I as a prognostic marker of leprosy reaction. Int J Lepr Other Mycobact Dis. 1998;66:356-64.
- [Google Scholar]
- Changes in circulating antibody levels to the major phenolic glycolipid during erythema nodosum leprosum in leprosy patients. Int J Lepr Other Mycobact Dis. 1985;53:211-7.
- [Google Scholar]
- IgM and IgG antibodies to phenolic glycolipid I from Mycobacterium leprae in leprosy: Insight into patient monitoring, erythema nodosum leprosum, and bacillary persistence. J Invest Dermatol. 1986;86:529-34.
- [CrossRef] [PubMed] [Google Scholar]






