Translate this page into:
Inflammasomes and diseases of the skin
2 VA Medical centre, Mather, Sacramento,
Correspondence Address:
S K Raychaudhuri
UC Davis/VA Medical Centre Sacramento, 10535 Hospital Way, Bldg# 807, Mather, CA 95655
| How to cite this article: Mitra A D, Schrock D, Raychaudhuri S P, Raychaudhuri S K. Inflammasomes and diseases of the skin. Indian J Dermatol Venereol Leprol 2012;78:394-402 |
Introduction
The inflammasome is a multiprotein complex that serves an important proinflammatory role in the innate immune system. The primary component of the inflammasome is the nucleotide oligomerization domain (NOD)-like receptors (NLR). There are over 20 known human NLRs and over 30 mice NLRs. [1],[2],[3],[4],[5],[6] It is unclear how many of these NLRs function as organizers of inflammasome formation. Till date, only four inflammasomes have been characterized: NLRP1, NLRP3, Ipaf and AIM2 inflammasomes. It has also been suggested that NLRP2 NLR, has the ability to form an inflammasome. [7] The NLRs that make up the central organizing component of the inflammasomes are divided into two major groups, the NLRPs and the NODs, and a third smaller group, the Ipafs. The NLRs all share some attributes in common. All NLRs have an active region containing a repetitive amino acid pattern rich in leucine [Figure - 1]. This region is termed leucine-rich repeat (LRR) and is important in functioning of NLRs as inflammasome organizers. LRR domain is believed to play a role in ligand-sensing/binding and regulating inflammasome activation. [8] Another active site common to all NLRs is NACHT (found in neuronal apoptosis inhibitory protein (NAIP), Class II transactivator (CIITA), incompatibility locus protein from Podospora anserina (HET-E), and telomerase-associated protein (TP-1) domain. The NACHT domain shows catalytic activity as an NTPase and is similar in structure and function to a group of apoptosis-associated proteins known as the STAND family of NTPases. [9] The crucial function of NACHT domain is believed to be its activity as a mediator of oligomerization, a function that has been documented in other members of STAND family. [10],[11] The final essential component of NLR is a binding domain capable of recruiting adapter proteins and/or effector caspases. This domain varies among the NLRs and can be a caspase recruitment domain (CARD), as seen in NLRP1 NLR or a PYD (pyrin domain). [7] Caspases involved in inflammasome signal cascade have been the topic of much recent research. Inflammatory caspases (caspases 1, 4 and 5), play a pivotal role in processing of inflammatory cytokines. Evidence suggests that, caspase 5 alone is not a very effective activator of IL-1β, synergistic activity of caspase 5 and caspase 1 seems to be very efficient at IL-1β processing, which may be indicative of the coactivation of caspases 1 and 5 that occurs in the NLRP1 inflammasome. It has also been suggested that caspase 5 may play a role in upstream regulation and activation of caspase 1. [12],[13] In a study by Salskov-Iverson, et al. [14] findings suggested that expression of caspase 5 is upregulated in lesional psoriatic skin as compared to nonlesional psoriatic, healthy skin and in other inflammatory skin diseases. Their findings also suggested that caspase 5 expression subsides along with psoriatic symptoms after anti-TNF-a treatment.
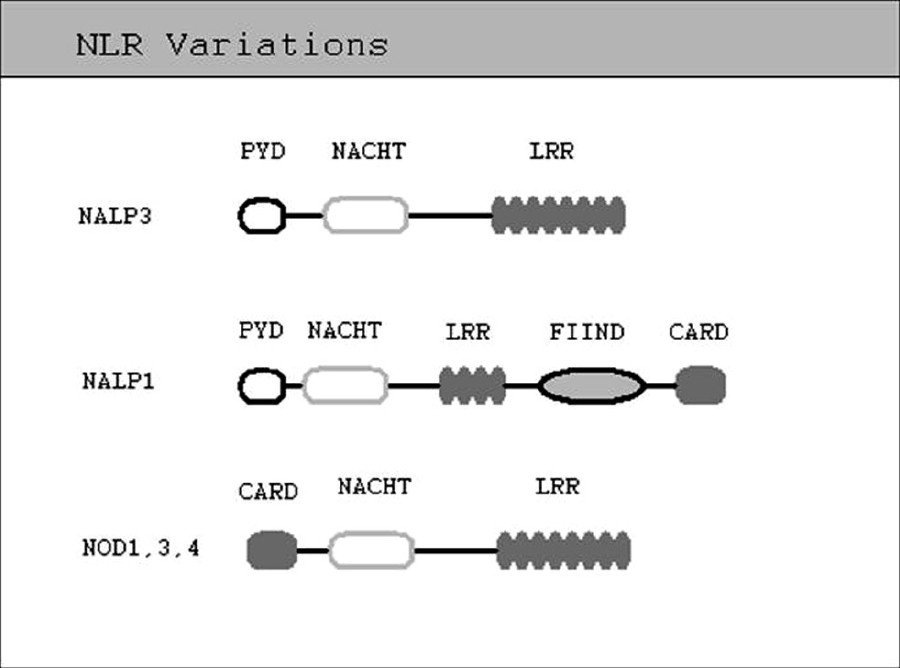 |
| Figure 1: Prototype of NLR structure showing the major interaction domains: The ligand sensing leucine - rich repeats, NACHT or the oligomerization domain, pyrin domain or caspase recruitment domain, acting as the effector domain and function to find domain |
Although inflammasome formation is quite established in the NLRP family and Ipaf family, the role of NOD family in inflammasome activity is still unclear. However, it has been shown that NOD signalsomes can activate inflammasome-related gene transcription through the NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathway. [7],[10],[11],[15] Evidence suggests that both NOD2 and NAIP, from the Ipaf family, are critical in inflammasome formation in some cases and may even be able to form inflammasomes on their own or through dimerization. [16],[17]
Recently, AIM2 inflammasome has been characterized. [18] It is the only inflammasome which functions without a member of the NLR family as an organizing component. [19],[20],[21],[22] AIM2 has been shown to be an activator of caspases 1 and IL-1β in the presence of endogenous and exogenous double-stranded DNA (ds DNA) 21 and is believed to be associated with systemic lupus erythematosis (SLE). [18] Recently, Dombrowski et al. [23] demonstrated that cytosolic DNA is an important disease-associated molecular pattern that can trigger AIM2 inflammasome and IL-1β activation in psoriasis.
Role of Inflammasomes in Inflammation
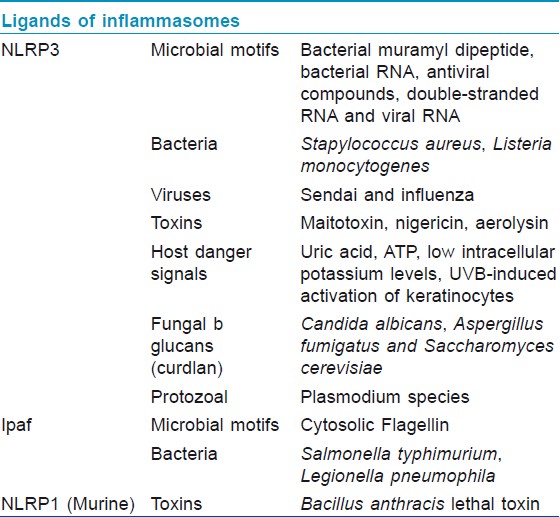
The primary role of inflammasome is to function as a detector of cytosolic signals of either tissue damage or the presence of a pathogen and to activate innate and adaptive immune responses. The cytosolic NLRs (and specifically, their LRR portions) have specificity for common pathogenic motifs known as pathogen-associated molecular patterns (PAMPs) [Table - 1] and endogenous signals of distress or damage known as danger-associated molecular patterns (DAMPs) such as excess K+ levels or cytosolic DNA. [24],[25],[26] Upon detection of its particular danger signal by LRR domain, the NLR initiates oligomerization via a CARD or PYD interaction with adapter proteins like ASC (Apoptosis-associated Speck-like Protein Containing a Caspase Recruitment Domain) which then recruit and activate effector inflammatory caspases. [10],[12] The activated caspases then cleave pro-IL-1β, pro-IL-18, and possibly pro-IL-33 into their active, proinflammatory forms [Figure - 2]. The activated cytokines are then secreted into the interstitial fluid to initiate inflammation. [7] IL-1β has an extremely powerful inflammatory and pyrogenic effect. It also induces the release of TNF, inducible nitric oxide synthase, COX-2 (Cyclooxygenase-2), prostaglandin E2, nitric oxide, type 2 phospholipase A, and even IL-1β itself depending on the type of cell. [27] IL-1β blockade has been used successfully to treat a number of inflammatory diseases such as; gout, familial Mediterranean fever (FMF), and several diseases in the family of inflammatory diseases known as cryopyrin-associated periodic syndrome (CAPS). [28] Additionally, the well-characterized inflammasomes (NLRP1, NLRP3, Ipaf, and AIM2) were all recently implicated in the process of pyroptosis in monocyte-derived cells. [20],[29],[30],[31],[32]
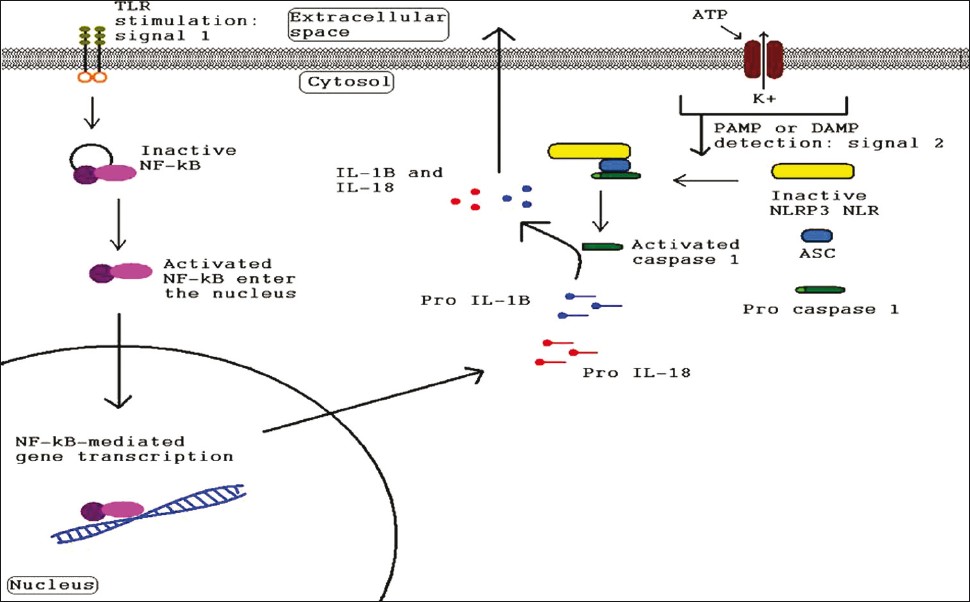 |
| Figure 2: Diagram illustrating the signals needed for inflammasome-related gene transcription and for inflammasome complex formation by pathogen-associated molecular patterns or damage-associated molecular patterns as well as the signal cascade leading to inflammatory cytokine secretion |
Pyroptotic Cell Death
Pyroptosis is a form of programmed cell death. Earlier it was termed as ′apoptosis′ due to morphologic similarities (apparent blebbing) and presence of DNA fragmentation, chromatin condensation and necessity for a caspase. [33],[34],[35],[36],[37] Subsequently it was shown to be different from apoptosis. [38],[39],[40] In contrast to apoptosis, it occurs after Caspase-1 activation, does not involve proapoptotic caspases (caspase 3, 8, 9) and does not cleave target proteins poly ADP ribose polymerase (PARP)1 and intercelluler adhesion protein D (ICAD). [36],[38],[41],[42],[43],[44]
Regulatory Role of Inflammasome in Confinement of Infection and Host Cell Repair
Gram negative bacterial infection lead to caspase-1 activation and IL-1β, IL-18 release. NLRC4/Ipaf deficient macrophages infected with salmonella are unable to activate caspase 1 and mature IL1β and IL18. Legionella, Pseudomonas and Shigella also induce caspase1 activation and phagosome maturation in a NLRC4 dependant manner. [45],[46] Activation of proinflammatory cytokines induce inflammation and confine infection. In addition, activation of caspase 1 leads to secretion of nuclear DAMP high-mobility group box 1 (HMGB1) from infected macrophages. [47] Decreased metabolic rate in infected cells due to loss of enzymatic activity of glyceraldehyde-3-phosphate dehydrogenase by Caspase 1 [48] limits intracellular pathogen replication and dictates host cells to undergo pyroptosis. Moreover, caspase 1 heals and repairs damage in host cells caused by bacterial pore forming toxins. Healing is regulated by activation of sterol regulatory element-binding protein 1 (SReBP1) and SReBP2 by NLRP3 and NLRC4 [Figure - 3]. Studies have also confirmed that maturation of procaspase 7 by NLRP3 and NLRC4 control intracellular replication of Legionella. [45]
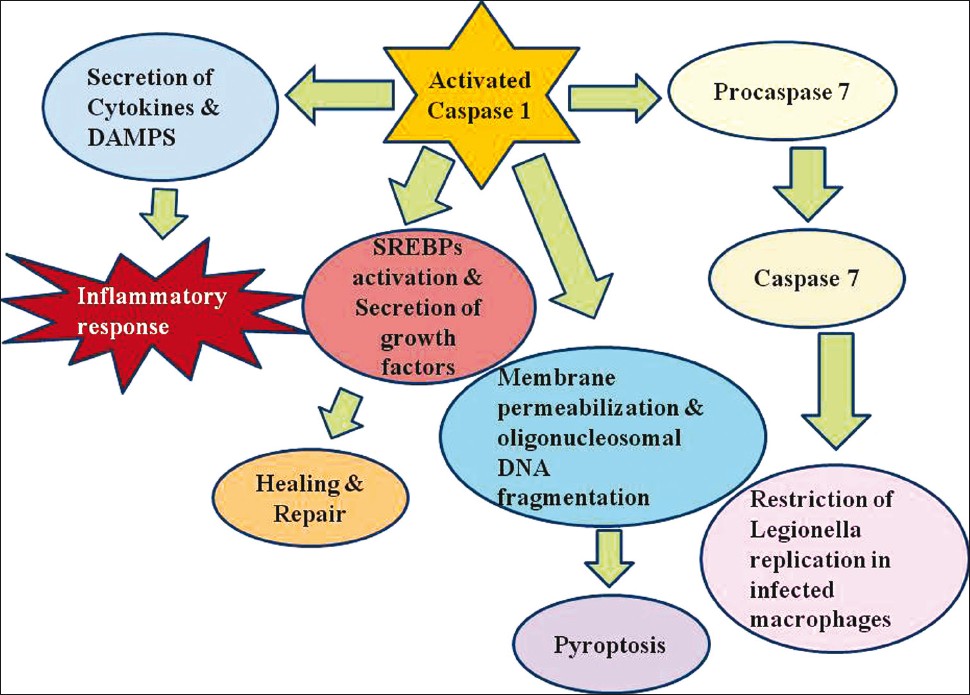 |
| Figure 3: Inflammasome assembly is triggered by pathogen assault of macrophages and dendritic cells, which ultimately leads to Caspase 1 activation. Activated caspase 1 regulates the release of numerous proinflammatory cytokines such as interleukin-1 β(IL-1β), IL-18 and IL-1a, and probably induces damage-associated molecular patterns such as high mobility group box 1 giving rise to inflammation. Caspase 1 also activates sterol regulatory element binding proteins, induces secretion of growth factors like fibroblast growth factor 2 and encourages repair and healing. Caspase 1 also prepares host cells to undergo pyroptosis, a form of programmed cell death involving membrane permeabilization and DNA fragmentation resulting in elimination of infected immune cells. Moreover caspase 7 activation by the inflammasome complex prevents bacterial replication in Legionella-infected macrophages |
Relationship Between Nlrp and Reactive Oxygen Species
The precise mechanism behind lysosomal activation of NLRP3 inflammasome is still indistinct. Earlier it was reported that crystal-induced activation of inflammasome was due to nicotinamide adenine dinucleotide phosphate (NADPH) oxidase mediated reactive oxygen species (ROS) production. [49],[50] However, some studies show that mutations in NADPH oxidase components and ineffective ROS production in patients with chronic granulomatous diseases still lead to inflammasome activation. [51] Recent reports suggest that mitochondrial ROS generation due to stress or defect in respiratory chain lead to upregulated IL-1β maturation through NLRP3 stimulation. [52],[53],[54] The precise mechanism of ROS-induced inflammasome activation is presumed to occur through interaction of thioredoxin-interacting protein (TXNIP) and NLRP3. [55]
Regulation of Inflammasome
Two known major areas of regulation on NLR are CARD domain and PYD domain. Blockade of CARD motif prevents efficient recruitment of effector caspases and, thereby, inhibits inflammasome cascade. [56],[57],[58],[59] Binding of PYD domain on NLR to ASC adapter protein is thought to be regulated by pyrin or other pyrin-only proteins (POPs). Pyrin and POPs bind directly to PYD site and inhibit inflammasome formation by competing for ASC. [60],[61] But pyrin also has a proinflammatory function and ability to actively assemble an inflammasome complex. [62] Guarda et al. demonstrated that NLRP1 and NLRP3 inflammasome activation could be blocked by memory and effector T cells. [63]
Nerve growth factor (NGF) and neuropeptides also induce IL-1β secretion and increases its mRNA expression in murine peritoneal macrophages and monocytes. [4] Raychaudhuri et al. demonstrated that IL-1β and other proinflammatory cytokines can induce NGF as well as its receptor expression in fibroblast like synoviocytes. [5] So it is a matter of debate as to whether NGF is responsible for proinflammatory cytokine secretion by activation of inflammasome cascade and whether the secreted cytokines induce secretion of NGF in a autocrine manner.
Expression of Inflammasome Components
Yin et al. showed NLR expression in lymphoid system, lymph nodes, spleen and thymus, peripheral blood, trachea, placenta, and brain with lymph nodes and trachea expressing the highest levels of NLRP1, and bone marrow and thymus tissue expressed the highest levels of NLRP3. [64] They reported a tiered system for determining inflammasome readiness of different tissues. First tier contains brain, blood, placenta and thymus. All these tissues constitutively express the components to form a fully functional inflammasome at any time. Second tier contains tissues (brain, pancreas, vascular, lymph nodes, trachea and spleen) that lack only one inflammasome component that can be readily induced. Lastly, third tier tissues (cardiac muscle) lack at least two (potentially inducible) inflammasome components. Potential inflammasome activity has been shown in many immune as well as nonimmune cells. Pancreatic β cells secrete IL-1β in response to chronic hyperglycemia which is presumed to be inflammasome dependant. [65] Keratinocytes express NOD1 and NOD2 and were able to produce IL-6 upon stimulation. [66] NLRP1 and NRLP3 were both detectable in neutrophils, monocytes, dendritic cells, B cells and T cells. [67],[68] However, macrophages and neutrophils only produced NLRP3 when induced with LPS. [68],[69] Nucleocytoplasmic staining [68] showed that NLRP1 was found in mononuclear cells in T-cell-related areas of tonsils, lymph nodes, spleen and also in Langerhans cells in mucosal tissues and in skin. Cytoplasmic staining revealed that epithelial cells in gastrointestinal tract, respiratory tract, endometrial and endocervical glands, gall bladder, and prostate all expressed detectable quantities of NLRP1. NLRP1 was also found in nuclei of pyramidal neurons and oligodendrocytes in brain as well as in cytoplasm of spermatogonia in testis. Cells that express cytoplasmic NLRP3 in detectable amounts are keratinocytes in oral, esophageal, and ectocervical mucosa, Hassall′s corpora in thymus and stratified epithelial cells in bladder and ureter.
Identification of Inflammasome Activity
Martinon et al., used an immunoprecipitation technique to confirm inflammasome oligomerization and caspase activation. [12] Target proteins (caspases 1, 2, 4, 5 and 9, NLRP1, Pycard, and Apaf) were tagged with a FLAG protein and then precipitated out of solution using anti-FLAG antibodies. These immunoprecipitation assays showed strong interactions between NLRP1 and caspases 5, as opposed to caspases 1, 2, 4, or 9. Inflammasome activation was also correlated to activation of caspases 1 and 5 which was demonstrated by detection of the cleavage products using western blot. Another indicator of inflammasome activation is simply to conduct a Western blot analysis of cell lysate, staining for NLR. Martinon et al. adapted a technique used by Cain et al. to determine whether there is inflammasome complex formation. [12],[70] Cell lysate was analyzed using Western blot staining with antibodies specific for the NLR whose mass was 165 kDa. Appearance of band at 700 kDa indicated NLR association with a large inflammasome complex. Similarly, staining with caspases 1 and 5 antibodies showed a shift from their theoretical weights to those associated with NLRP1 inflammasome.
Using mice or human cells deficient in one or more inflammasome components also appeared to be an effective way to be assured of their activity. Mice deficient in either one or both of NLRP3 inflammasome components NLRP3 and ASC were unable to mount an effective immune response. [71]
PCR and subsequent Western blot was used for mRNA and protein expression of caspases, ASC and NLRs. [14]
Role of the Inflammasome in Cutaneous Autoimmune and Autoinflammatory Disease
Studies linking inflammasomes to adjuvanicity and inflammasome product blockade to remission of symptoms in gout, FMF, Muckle-Wells syndrome (MWS), familial cold autoinflammatory syndrome (FCAS) suggest a central role of inflammasomes and their accessory caspases in autoimmune and autoinflammatory diseases. [14],[72],[73],[74],[75],[76] Here, we describe some cutaneous diseases like contact dermatitis, psoriasis, vitiligo and atopic dermatitis and how inflammasomes may be participating in their pathophysiology.
CAPS and autoimmune cold urticaria
CAPS family of diseases include MWS, FCAS, and neonatal-onset multisystem inflammatory disease (NOMID). They are associated with missense mutations in NACHT oligomerization-regulating domain of NLRP3 NLR. [74],[78] These mutations, are responsible for upregulation of NLRP3 inflammasome and overproduction of mature IL-1β. [31] Nakamura et al. [79] demonstrated NLRP3-associated mutations in the pathophysiology of MWS. Mast cells (MCs) are concentrated in areas commonly affected by CAPS-associated urticaria; the skin, the joints and the central nervous system. [80] They showed that MCs from healthy tissue constitutively express caspase 1 and ASC. Moreover, studies show that anti-IL-1β therapy resulted in successful treatment of CAPS. [73],[81],[82]
Contact dermatitis
It has been reported that induction of epidermal hypersensitivity by chemical irritant is inflammasome-dependant. [83] Cavani et al. showed that inflammasome was involved in development of contact hypersensitivity, a T-cell-mediated reaction, and therefore had been exerting influence over the adaptive immune system. Inflammasome participation in this reaction is also implied by the discovery that sensitization phase of the hypersensitivity reaction is dependent on activated caspases-1, IL-1β, and IL-18. [84],[85],[86],[87] Furthermore, a study was conducted using mice deficient for ASC and NLRP3 in which the mice displayed a reduced capacity to generate a hypersensitivity reaction to certain irritants. [71] This demonstrated the importance of inflammasome in overall hypersensitivity response and the fact that these immune-deficient mice were able to mount normal elicitation phase contact hypersensitivity reactions upon innoculation with presensitized T-cells solidified the hypothesis that the inflammasome was only crucial in the sensitization phase. [88] In other words, the phase of the hypersensitivity reaction in which antigen-presenting cells (APCs) take up antigen and migrate from skin to lymph nodes to present it to naïve T-cells is mediated by inflammasome activity.
Psoriasis
Experimental results suggest that caspase 5, an effector caspase in the NLRP1 inflammasome, is a powerful promoter of psoriatic pathology. [14] The results showed that there was increased mRNA expression of caspase 5 and a smaller, but still significant, upregulation of ASC (a NLRP1 inflammasome component) in lesional psoriatic tissue. Caspase 5 upregulation is showed to be an indicator of high immunogenic activity and its expression is not increased in other inflammatory skin diseases (contact hypersensitivity and atopic dermatitis). Moreover, psoriasis patients have lesser cutaneous infections compared to other inflammatory skin diseases implying inflammasome hyperactivity to be a major contributing factor. Recently Dombrowski et al., demonstrated that cytosolic DNA is an important disease-associated molecular pattern that can trigger AIM2 inflammasome and IL-1β activation in psoriasis, providing new potential targets for treatment of this chronic skin disease.
Vitiligo
The precise role of the inflammasome in vitiligo and vitiligo-associated autoimmune diseases is still a point of speculation. It was shown by Jin et al. [89] that mutations in NACHT region of the NLRP1 NLR gene, as well as a mutation in the regulatory region of that gene, were correlated to expression of vitiligo phenotype. Taïeb [90] suggested that the pathological inflammasome activity in vitiligo is altering adaptive immunity by prioritizing melanocyte clearance. This hypothesis is supported in part by a finding of slightly elevated T-cell infiltrates in skin of vitiligo patients. [91] Furthermore, vitiligo is known to often coincide with autoimmune diseases such as rheumatoid arthritis, SLE, and diabetes which, by extension, suggest a role for the NLRP1 inflammasome in these diseases. [89]
Atopic dermatitis
Atopic dermatitis is a complex disease with environmental factors, impaired recruitment of innate immune cells and decreased anti-microbial peptide secretion contributing both to autoinflammatory pathology as well as susceptibility to infection. [92] NLRP1, NLRP3, NOD1, and NOD2 are all believed to be responsible for innate detection of bacterial motifs in pathogens commonly associated with atopic dermatitis infections such as certain Gram-negative bacteria and Staphylococcus aureus. [93] The inability of patients with atopic dermatitis to cope with pathogens suggests that some deficiency in the inflammasome signal cascade. Additionally, the gene encoding NOD1 is located in a region that has been linked with atopy and mutations in NOD1, NOD2, and NLRP12 have all been correlated to diagnoses of atopic dermatitis. [94],[95],[96] Nakanishi et al. [97] illustrate the importance of IL-18 in the pathogenesis of atopic dermatitis. IL-18 influences adaptive immune response by stimulating Th1 cells to "super Th1 cells" that produce both Th1 and Th2 cytokines. [98] These "super Th1 cells" were identified in mice with induced atopic dermatitis. IL-18 was also demonstrated to be present and originating from keratinocytes. IL-18 blockade successfully prevented both development of induced atopic dermatitis and generation of "super Th1 cells". [99]
Conclusions
In this review, we summarized the role of inflammasomes and their activation in cellular damage or stress. Activation of inflammasomes require ′priming′ with lipopolysaccharide and a second signal in the form of low intracellular potassium, increased mitochondrial ROS levels, lysosomal destabilization, or other unknown factors. A better understanding about the regulatory role of inflammasomes in host defense, autoinflammatory conditions and cell repair will open new therapeutic targets for infectious and inflammatory diseases. The role of inflammasome in the pathogenesis of gout and rheumatoid arthritis has reached the point where therapeutic targets are being identified and tested in humans. Some unanswered questions regarding inflammasome activation and function still remain, which over the years is expected to be solved.
| 1. |
Kannan Y, Ushio H, Koyama H, Okada M, Oikawa M, Yoshihara T, et al. 2.5S Nerve growth factor enhances the survival, phagocytosis, and superoxide production of murine neutrophils. Blood 1991;77:1320-5.
[Google Scholar]
|
| 2. |
Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008;123:398-410.
[Google Scholar]
|
| 3. |
Raychaudhuri SP, Raychaudhuri SK, Atkuri KR, Herzenberg LA, Herzenberg LA. Nerve growth factor: A key local regulator in the pathogenesis of inflammatory arthritis. Arthritis Rheum 2011;63:3243-52.
[Google Scholar]
|
| 4. |
Susaki Y, Shimizu S, Katakura K, Watanabe N, Kawamoto K, Matsumoto M, et al. Functional properties of murine macrophages promoted by nerve growth factor. Blood 1996;88:4630-7.
[Google Scholar]
|
| 5. |
Raychaudhuri SP, Raychaudhuri SK. The regulatory role of nerve growth factor and its receptor system in fibroblast-like synovial cells. Scand J Rheumatol 2009;38:207-15.
[Google Scholar]
|
| 6. |
Sidiropoulos PI, Goulielmos G, Voloudakis GK, Petraki E, Boumpas DT. Inflammasomes and rheumatic diseases: Evolving concepts. Ann Rheum Dis 2008;67:1382-9.
[Google Scholar]
|
| 7. |
Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol 2009;27:229-65.
[Google Scholar]
|
| 8. |
Bella J, Hindle KL, McEwan PA, Lovell SC. The leucine-rich repeat structure. Cell Mol Life Sci 2008;65:2307-33.
[Google Scholar]
|
| 9. |
Leipe DD, Koonin EV, Aravind L. STAND, a class of P-loop NTPases including animal and plant regulators of programmed cell death: Multiple, complex domain architectures, unusual phyletic patterns, and evolution by horizontal gene transfer. J Mol Biol 2004;343:1-28.
[Google Scholar]
|
| 10. |
Martinon F, Tschopp J. Inflammatory caspases: Linking an intracellular innate immune system to autoinflammatory diseases. Cell 2004;117:561-74.
[Google Scholar]
|
| 11. |
Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell 2007;25:713-24.
[Google Scholar]
|
| 12. |
Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002;10:417-26.
[Google Scholar]
|
| 13. |
Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: Master switches of inflammation. Cell Death Differ 2007;14:10-22.
[Google Scholar]
|
| 14. |
Salskov-Iversen ML, Johansen C, Kragballe K, Iversen L. Caspase-5 expression is upregulated in lesional psoriatic skin. J Invest Dermatol 2011;131:670-6.
[Google Scholar]
|
| 15. |
Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol 2001;19:331-73.
[Google Scholar]
|
| 16. |
Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: Dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol 2007;29:275-88.
[Google Scholar]
|
| 17. |
Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, et al. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol 2008;10:1-8.
[Google Scholar]
|
| 18. |
Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol 2009;19:455-64.
[Google Scholar]
|
| 19. |
Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, Jahn H, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol 2009;10:266-72.
[Google Scholar]
|
| 20. |
Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009;458:509-13.
[Google Scholar]
|
| 21. |
Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009;458:514-8.
[Google Scholar]
|
| 22. |
Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 2009;323:1057-60.
[Google Scholar]
|
| 23. |
Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Göss C, Anz D, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med 2011;3:82ra38.
[Google Scholar]
|
| 24. |
Janeway CA Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 1989;54:1-13.
[Google Scholar]
|
| 25. |
Matzinger P. The danger model: A renewed sense of self. Science 2002;296:301-5.
[Google Scholar]
|
| 26. |
Seong SY, Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 2004;4:469-78.
[Google Scholar]
|
| 27. |
Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009;27:519-50.
[Google Scholar]
|
| 28. |
Goldbach-Mansky R, Kastner DL. Autoinflammation: The prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses. J Allergy Clin Immunol 2009;124:1141-9.
[Google Scholar]
|
| 29. |
Kufer TA, Sansonetti PJ. Sensing of bacteria: NOD a lonely job. Curr Opin Microbiol 2007;10:62-9.
[Google Scholar]
|
| 30. |
Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol 2009;7:99-109.
[Google Scholar]
|
| 31. |
Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1 beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 2004;20:319-25.
[Google Scholar]
|
| 32. |
Dowds TA, Masumoto J, Chen FF, Ogura Y, Inohara N, Núñez G. Regulation of cryopyrin/Pypaf1 signaling by pyrin, the familial Mediterranean fever gene product. Biochem Biophys Res Commun 2003;302:575-80.
[Google Scholar]
|
| 33. |
Chen LM, Kaniga K, Galán JE. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol 1996;21:1101-15.
[Google Scholar]
|
| 34. |
Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci U S A 1996;93:9833-8.
[Google Scholar]
|
| 35. |
Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1 beta-converting enzyme, caspase 1, is activated during Shigella flexneriinduced apoptosis in human monocyte derived macrophages. Infect Immun 1997;65:5165-70.
[Google Scholar]
|
| 36. |
Hilbi H, Moss JE, Hersh D, Chen Y, Arondel J, Banerjee S, et al. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem 1998;273:32895-900.
[Google Scholar]
|
| 37. |
Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A 1999;96:2396-401.
[Google Scholar]
|
| 38. |
Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol 2000;38:31-40.
[Google Scholar]
|
| 39. |
Watson PR, Gautier AV, Paulin SM, Bland AP, Jones PW, Wallis TS. Salmonella enterica serovars Typhimurium and Dublin can lyse macrophages by a mechanism distinct from apoptosis. Infect Immun 2000;68:3744-7.
[Google Scholar]
|
| 40. |
Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol 2001;9:113-4.
[Google Scholar]
|
| 41. |
Jesenberger V, Procyk KJ, Yuan J, Reipert S, Baccarini M. Salmonella-induced caspase-2 activation in macrophages: A novel mechanism in pathogen-mediated apoptosis. J Exp Med 2000;192:1035-46.
[Google Scholar]
|
| 42. |
Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, et al. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics 2008;7:2350-63.
[Google Scholar]
|
| 43. |
Chen Y, Smith MR, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J 1996;15:3853-60.
[Google Scholar]
|
| 44. |
Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 2006;8:1812-25.
[Google Scholar]
|
| 45. |
Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, et al. The Birc 1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol 2006;7:318-25.
[Google Scholar]
|
| 46. |
Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Núñez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol 2007;37:3030-9.
[Google Scholar]
|
| 47. |
Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol 2010;185:4385-92.
[Google Scholar]
|
| 48. |
Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem 2007;282:36321-9.
[Google Scholar]
|
| 49. |
Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008;320:674-7.
[Google Scholar]
|
| 50. |
Schroder K, Tschopp J. The inflammasomes. Cell 2010;140:821-32.
[Google Scholar]
|
| 51. |
van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, van der Meer JW, et al. Reactive oxygen species-independent activation of the IL-1 beta inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci U S A 2010;107:3030-3.
[Google Scholar]
|
| 52. |
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011;469:221-5.
[Google Scholar]
|
| 53. |
West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011;472:476-80.
[Google Scholar]
|
| 54. |
Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 2011;12:222-30.
[Google Scholar]
|
| 55. |
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 2010;11:136-40.
[Google Scholar]
|
| 56. |
Humke EW, Shriver SK, Starovasnik MA, Fairbrother WJ, Dixit VM. ICEBERG: A novel inhibitor of interleukin-1 beta generation. Cell 2000;103:99-111.
[Google Scholar]
|
| 57. |
Lee SH, Stehlik C, Reed JC. Cop, a caspase recruitment domain-containing protein and inhibitor of caspase-1 activation processing. J Biol Chem 2001;276:34495-500.
[Google Scholar]
|
| 58. |
Druilhe A, Srinivasula SM, Razmara M, Ahmad M, Alnemri ES. Regulation of IL-1 beta generation by Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ 2001;8:649-57.
[Google Scholar]
|
| 59. |
Lamkanfi M, Denecker G, Kalai M, D'hondt K, Meeus A, Declercq W, et al. INCA, a novel human caspase recruitment domain protein that inhibits interleukin-1 beta generation. J Biol Chem 2004;279:51729-38.
et al. INCA, a novel human caspase recruitment domain protein that inhibits interleukin-1 beta generation. J Biol Chem 2004;279:51729-38.'>[Google Scholar]
|
| 60. |
Johnston JB, Barrett JW, Nazarian SH, Goodwin M, Ricciuto D, Wang G, et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 2005;23:587-98.
[Google Scholar]
|
| 61. |
Chae JJ, Wood G, Masters SL, Richard K, Park G, Smith BJ, et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1 beta production. Proc Natl Acad Sci U S A 2006;103:9982-7.
[Google Scholar]
|
| 62. |
Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S, et al. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ 2006;13:236-49.
[Google Scholar]
|
| 63. |
Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, et al. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature 2009;460:269-73.
[Google Scholar]
|
| 64. |
Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, et al. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol 2009;22:311-22.
[Google Scholar]
|
| 65. |
Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: A sensor for metabolic danger? Science 2010;327:296-300.
[Google Scholar]
|
| 66. |
Song PI, Park YM, Abraham T, Harten B, Zivony A, Neparidze N, et al. Human keratinocytes express functional CD14 and toll-like receptor 4. J Invest Dermatol 2002;119:424-32.
[Google Scholar]
|
| 67. |
Anderson JP, Mueller JL, Rosengren S, Boyle DL, Schaner P, Cannon SB, et al. Structural, expression, and evolutionary analysis of mouse CIAS1. Gene 2004;338:25-34.
[Google Scholar]
|
| 68. |
Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem 2007;55:443-52.
[Google Scholar]
|
| 69. |
Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 2006;24:317-27.
[Google Scholar]
|
| 70. |
Cain K, Brown DG, Langlais C, Cohen GM. Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase-activating complex. J Biol Chem 1999;274:22686-92.
[Google Scholar]
|
| 71. |
Watanabe H, Gaide O, Pétrilli V, Martinon F, Contassot E, Roques S, et al. Activation of the IL-1 beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol 2007;127:1956-63.
[Google Scholar]
|
| 72. |
Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1 beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 2007;8:942-9.
[Google Scholar]
|
| 73. |
Hawkins PN, Lachmann HJ, Mc Dermott MF. Interleukin-1-receptor antagonist in the Muckle-Wells syndrome. N Engl J Med 2003;348:2583-4.
[Google Scholar]
|
| 74. |
Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet 2001;29:301-5.
[Google Scholar]
|
| 75. |
Rosengren S, Mueller JL, Anderson JP, Niehaus BL, Misaghi A, Anderson S, et al. Monocytes from familial cold autoinflammatory syndrome patients are activated by mild hypothermia. J Allergy Clin Immunol 2007;119:991-6.
[Google Scholar]
|
| 76. |
Rao DA, Tracey KJ, Pober JS. IL-1 alpha and IL-1 beta are endogenous mediators linking cell injury to the adaptive alloimmune response. J Immunol 2007;179:6536-46.
[Google Scholar]
|
| 77. |
Braun-Falco M, Ruzicka T. Skin manifestations in autoinflammatory syndromes. J Dtsch Dermatol Ges 2011;9:232-46.
[Google Scholar]
|
| 78. |
Aganna E, Martinon F, Hawkins PN, Ross JB, Swan DC, Booth DR, et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum 2002;46:2445-52.
[Google Scholar]
|
| 79. |
Nakamura Y, Kambe N, Saito M, Nishikomori R, Kim YG, Murakami M, et al. Mast cells mediate neutrophil recruitment and vascular leakage through the NLRP3 inflammasome in histamine-independent urticaria. J Exp Med 2009;206:1037-46.
[Google Scholar]
|
| 80. |
Neven B, Prieur AM, Quartier dit Maire P. Cryopyrinopathies: Update on pathogenesis and treatment. Nat Clin Pract Rheumatol 2008;4:481-9.
[Google Scholar]
|
| 81. |
Hoffman HM, Rosengren S, Boyle DL, Cho JY, Nayar J, Mueller JL, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet 2004;364:1779-85.
[Google Scholar]
|
| 82. |
Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1 beta inhibition. N Engl J Med 2006;355:581-92.
[Google Scholar]
|
| 83. |
Cavani A, De Pità O, Girolomoni G. New aspects of the molecular basis of contact allergy. Curr Opin Allergy Clin Immunol 2007;7:404-8.
[Google Scholar]
|
| 84. |
Shornick LP, De Togni P, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, et al. Mice deficient in IL-1 beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. J Exp Med 1996;183:1427-36.
[Google Scholar]
|
| 85. |
Zepter K, Häffner A, Soohoo LF, De Luca D, Tang HP, Fisher P, et al. Induction of biologically active IL-1 beta-converting enzyme and mature IL-1 beta in human keratinocytes by inflammatory and immunologic stimuli. J Immunol 1997;159:6203-8.
[Google Scholar]
|
| 86. |
Antonopoulos C, Cumberbatch M, Dearman RJ, Daniel RJ, Kimber I, Groves RW. Functional caspase-1 is required for Langerhans cell migration and optimal contact sensitization in mice. J Immunol 2001;166:3672-7.
[Google Scholar]
|
| 87. |
Wang B, Feliciani C, Howell BG, Freed I, Cai Q, Watanabe H, et al. Contribution of Langerhans cell-derived IL-18 to contact hypersensitivity. J Immunol 2002;168:3303-8.
[Google Scholar]
|
| 88. |
Yazdi AS, Ghoreschi K, Röcken M. Inflammasome activation in delayed-type hypersensitivity reactions. J Invest Dermatol 2007;127:1853-5.
[Google Scholar]
|
| 89. |
Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med 2007;356:1216-25.
[Google Scholar]
|
| 90. |
Taïeb A. NALP1 and the inflammasomes: Challenging our perception of vitiligo and vitiligo-related autoimmune disorders. Pigment Cell Res 2007;20:260-2.
[Google Scholar]
|
| 91. |
van den Wijngaard R, Wankowicz-Kalinska A, Le Poole C, Tigges B, Westerhof W, Das P. Local immune response in skin of generalized vitiligo patients. Destruction of melanocytes is associated with the prominent presence of CLA+ T cells at the perilesional site. Lab Invest 2000;80:1299-309.
[Google Scholar]
|
| 92. |
De Benedetto A, Agnihothri R, Mc Girt LY, Bankova LG, Beck LA. Atopic dermatitis: A disease caused by innate immune defects? J Invest Dermatol 2009;129:14-30.
[Google Scholar]
|
| 93. |
Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol 2004;41:1099-108.
[Google Scholar]
|
| 94. |
Kabesch M, Peters W, Carr D, Leupold W, Weiland SK, von Mutius E. Association between polymorphisms in caspase recruitment domain containing protein 15 and allergy in two German populations. J Allergy Clin Immunol 2003;111:813-7.
[Google Scholar]
|
| 95. |
Weidinger S, Klopp N, Rummler L, Wagenpfeil S, Novak N, Baurecht HJ, et al. Association of NOD1 polymorphisms with atopic eczema and related phenotypes. J Allergy Clin Immunol 2005;116:177-84.
[Google Scholar]
|
| 96. |
Macaluso F, Nothnagel M, Parwez Q, Petrasch-Parwez E, Bechara FG, Epplen JT, et al. Polymorphisms in NACHT-LRR (NLR) genes in atopic dermatitis. Exp Dermatol 2007;16:692-8.
[Google Scholar]
|
| 97. |
Nakanishi K, Tsutsui H, Yoshimoto T. Importance of IL-18-induced super Th1 cells for the development of allergic inflammation. Allergol Int 2010;59:137-41.
[Google Scholar]
|
| 98. |
Terada M, Tsutsui H, Imai Y, Yasuda K, Mizutani H, Yamanishi K, et al. Contribution of IL-18 to atopic-dermatitis-like skin inflammation induced by Staphylococcus aureus product in mice. Proc Natl Acad Sci U S A 2006;103:8816-21.
[Google Scholar]
|
| 99. |
Nakano H, Tsutsui H, Terada M, Yasuda K, Matsui K, Yumikura-Futatsugi S, et al. Persistent secretion of IL-18 in the skin contributes to IgE response in mice. Int Immunol 2003;15:611-21.
[Google Scholar]
|
Fulltext Views
3,967
PDF downloads
3,566





