Translate this page into:
Infliximab: Efficacy in psoriasis
Correspondence Address:
Shehnaz Arsiwala
Saifee Hospital, 13/15 Maharishi Karve Road, Mumbai, Maharashtra
India
| How to cite this article: Arsiwala S. Infliximab: Efficacy in psoriasis. Indian J Dermatol Venereol Leprol 2013;79:25-34 |
Abstract
Moderate to severe psoriasis often needs to be addressed with standard disease modifying therapies such as methotrexate, cyclosporine, acitretin or ultraviolet radiation, which have their potential benefits and limitations. The tumor necrosis factor-alpha (TNF-α) is elevated in psoriatic plaques compared to non lesional skin as well as in the plasma of patients with moderate to severe psoriasis. Infliximab, a TNF-α blocker, has been recommended for the treatment of moderate to severe plaque psoriasis in adults who have failed to respond to these therapies or who cannot tolerate them. Its specific action on the bound and membrane forms of the pro-inflammatory cytokine TNF-α has made it the molecule of choice for obtaining quicker and longer remission in recalcitrant cases. However, the widespread use of infliximab in the Indian subcontinent is limited by its cost. This article reviews the international guidelines for use of infliximab, its dosage patterns, and efficacy in chronic plaque psoriasis, nail psoriasis, erythrodermic psoriasis, and pustular psoriasis as well as Indian experience.Introduction
Psoriasis is a chronic inflammatory, immune-mediated, cutaneous disorder with multisystem comorbidities. Moderate to severe psoriasis is sometimes unresponsive or only partly controlled by standard systemic therapies like methotrexate (MTX), acitretin, cyclosporine, psoralen + ultraviolet A light (PUVA), and ultraviolet B light (UVB), which have their own limitations. Recombinant DNA (rDNA) technology enables development of biologics from cells or tissues. These molecules have target specific pharmacologic activity. rDNA technology has enabled the development of biologics that have specific pharmacologic activity offering an alternative to the way we treat severe psoriatic diseases. [1],[2] [Table - 1] lists the biologics available for psoriasis. The biologics available in India for the treatment of psoriasis are the tumor necrosis factor-alpha (TNF-α) antagonists-infliximab and etanercept. Infliximab is a chimeric human-murine monoclonal antibody (25% mouse-derived protein), whereas adalimumab is fully human. Etanercept is a genetically engineered fusion protein composed of a dimer of the extracellular portions of human TNFR2 (p75) fused to the Fc domain of human immunoglobulin G1 (IgG1). [1],[2] This article reviews the use of infliximab in chronic plaque, erythrodermic, and pustular psoriasis; its usage pattern; efficacy; and international guidelines as well as Indian experience.

Need for Target Specific Therapies in Psoriasis
Psoriasis is considered an inflammatory autoimmune disorder that is T cell mediated. [3] The inflammatory T helper 1 (Th1) cytokines TNF-α, as well as interleukin-12 (IL-12) and IL-23, are necessary for its induction and maintenance of disease activity in psoriasis. [4],[5],[6],[7] TNF-α is elevated in psoriatic plaques compared to non lesional skin and plasma of patients with psoriasis. [8] The serum TNF-α concentration rises with disease activity and decreases with increasing therapeutic response. [9]
Infliximab
Chemical structure
Infliximab has a variable murine fragment antigen binding (Fab) component (25%) and a constant human component (Fc) of human IgG1 (75%), thus making it a mouse/human IgG1 isotype chimeric monoclonal antibody as shown in [Figure - 1]. It binds specifically and with high affinity to human TNF-α and weighs 149,100 Da. [10],[11]
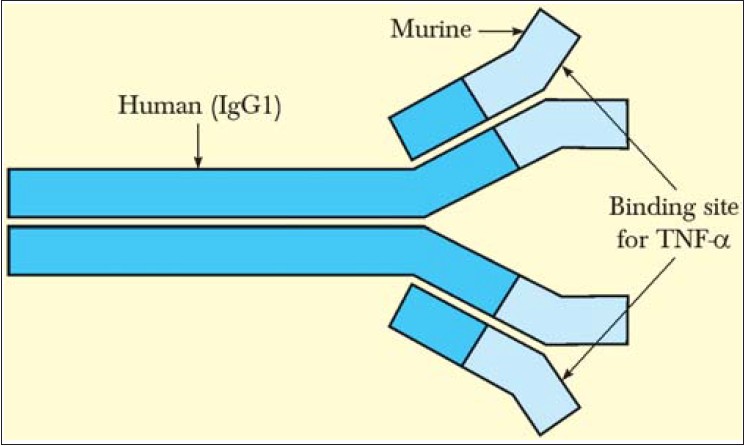 |
| Figure 1: Infliximab structure |
Mechanism of action
Infliximab can competitively block TNF-α by binding to its soluble as well as the bound transmembrane form irreversibly. [11] It can bind both monomeric and trimeric TNF-α, in the ratio of 2:1, thus forming multimeric complexes as illustrated in [Figure - 2] and [Figure - 3]. This differentiates it from etanercept, which can block only soluble TNF-α, binding it reversibly. Once bound, the likelihood of dissociation of TNF-α from infliximab is significantly less, enabling the molecule to achieve rapid action and substantial improvement of severe psoriasis. [12] After infliximab-TNF-α binding in the plasma and the plaques, the TNF-α cannot activate the tissue receptors. Infliximab has additional depleting effects on TNF-α producing cells causing their apoptosis by complement lysis as well as antibody-dependent cellular cytotoxicity; thus not only is the TNF-α neutralized but it cannot be biologically activated again. [13]
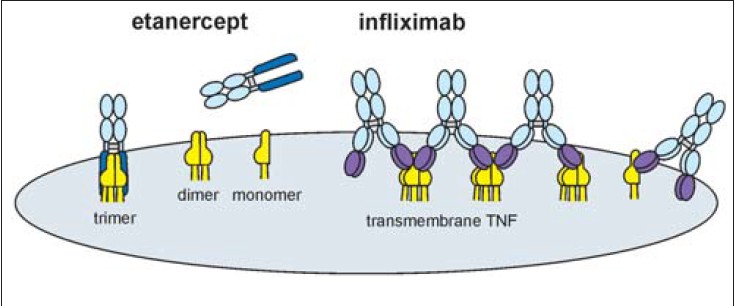 |
| Figure 2: Infliximab binding to TNF-α receptors. Source: *Journal of Pharmacology and Experimental Therapeutics |
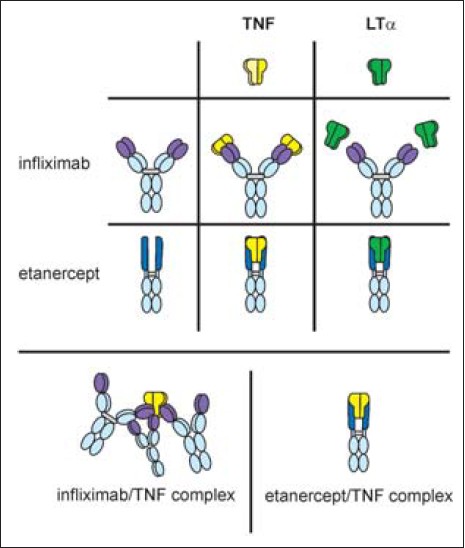 |
| Figure 3: Difference in etanercept-TNF complex and infliximab-TNF complex. Source: *Journal of Pharmacology and Experimental Therapeutics |
Pharmacokinetics
Infliximab is available as 100 mg of preservative free powder that needs reconstitution before infusion. It is administered in the dose of 3, 5 or 10 mg/kg, diluted in 500 ml of normal saline, by intravenous infusion over 2 h, repeated at 2 and 6 weeks (the induction phase) and thereafter every 8 weeks (the maintenance phase). A mean maximum serum concentration (C max ) of 118 mg/ml is achieved after a single infusion of 5 mg/kg. The half-life is about 7-10 days. Post infusion, infliximab can be detected in the serum for up to 28 weeks depending on the dose and the duration of therapy. [14] The elimination routes for infliximab have not been fully identified. [15] Infliximab crosses the placenta and has a long half-life, but is not detected in the breast milk. [15]
Indications
In the United States and Europe, infliximab has been approved for the indications of rheumatoid arthritis, Crohn′s disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. In Japan, it has also been approved for intractable uveitis in Behçet′s disease. The licensed indication for its use is moderate to severe plaque psoriasis and in patients who have failed to respond to, have a contraindication to or are intolerant of other systemic therapies including cyclosporine, methotrexate, and PUVA. Some off-label uses have been reported [Table - 2]. [16]
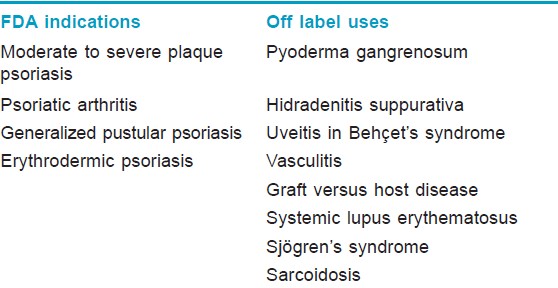
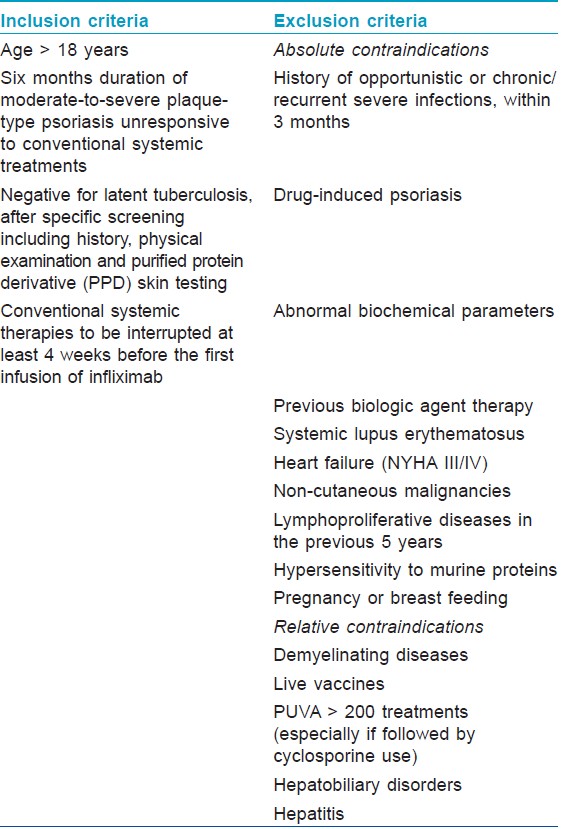
Eligibility Criteria and recommended guidelines for infliximab in psoriasis
Review of various studies from 1990 to 2011 have shown infliximab′s efficacy in moderate to severe chronic plaque psoriasis with doses of 3, 5 or 10mg/kg body weight, and guidelines of criteria for patient selection and exclusion are very well defined. Biologic therapy is recommended only in recalcitrant cases of moderate to severe plaque psoriasis or variants of generalized psoriasis not responsive to conventional antipsoriatic therapies. The indications and the inclusion and exclusion criteria for the use of infliximab in psoriasis recommended by various guidelines [9],[15],[17],[18],[19],[20],[21],[22],[23],[24] are summarized in [Table - 3] and [Table - 4].

Efficacy and onset of action
A favorable response is defined as either 50% or greater reduction in baseline Psoriasis Area Severity Index (PASI 50) or PASI 75, that is, improvement of 75% from baseline PASI score in chronic recalcitrant plaque psoriasis. Some trials also reported patients with a PASI 90 response. [9],[15],[17],[18],[19],[25] Body surface area (BSA) affected should be used when PASI scoring is not applicable as with severe disease, defined as > 10% BSA affected. [25]
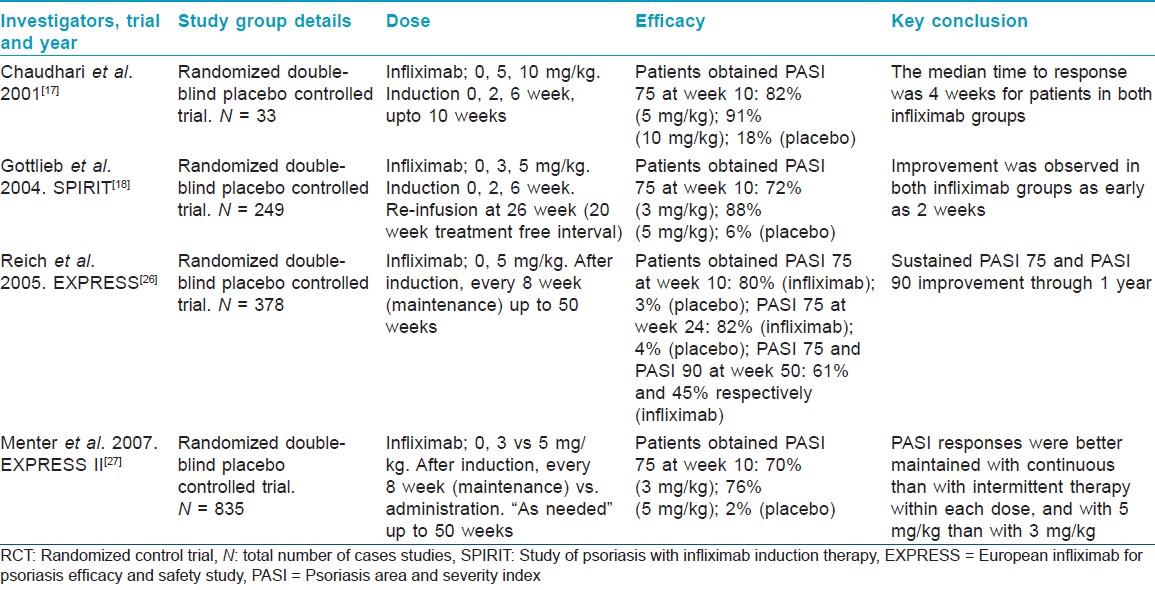
Many trials have demonstrated the efficacy of infliximab in moderate to severe chronic plaque psoriasis. [9],[17],[18],[19],[26],[27] [Table - 5] highlights the significant infliximab studies in moderate to severe psoriasis. Study by Chaudhari et al. [17] was a induction study, followed by the larger SPIRIT (Study of psoriasis with infliximab induction therapy) trial. [18] Reich et al. [19] conducted the long term maintenance therapy trial whereas, EXPRESS II (European infliximab for psoriasis efficacy and safety study) trial monitored continuous vs intermittent treatment. These extensive data studies form a basis for infliximab induction in psoriasis. [27] According to the British and European guidelines, four randomized control trials (RCT) of very good methodological quality indicate the high efficacy of infliximab in chronic plaque psoriasis, with significant response seen within 2 weeks of infusion and the optimum response by week 10. [15],[23]
Two trials have addressed the long-term efficacy of infliximab in psoriasis. In a trial with infliximab 5, 10 mg/kg or placebo, patients who improved were evaluated for relapse (defined as loss of half the PASI improvement at week 10) at week 10-26. These patients and placebo non-responders were treated with infliximab 5 or 10 mg/kg and reassessed at week 26. Out of the 33 patients, 29 received infliximab after week 26. PASI response was maintained in 40% with the 5 mg/kg dose and in 73% with the 10 mg/kg dose. [18] Significant long term PASI 75 response at 6 months is also highlighted in the EXPRESS trial. [18],[28] Achieving stable serum infliximab levels correlated with the maintenance of clinical response; patients who achieved a PASI 75 response by week 50 had infliximab levels of 1 mg/mL or more in their serum. [19] Assessment of serum infliximab concentration at week 8 after treatment, the PASI 75 response rate in patients with a serum infliximab concentration of < 0.1 mg/mL, 0.1 to < 1 mg/mL, and 1 to < 10 mg/mL was 60.0% (6/10), 71.4% (5/7), and 95.7% (22/23), respectively. [29]
The median trough concentration of infliximab was observed to be more than the limit of quantitation (0.1 mg/mL) in the infliximab group, and a steady-state concentration was maintained through week 70. The PASI 75 response rate increased with an increase in serum infliximab concentrations. At 4 weeks after the last infusion, 50% or more of the patients with a serum infliximab concentration of 0.1 mg/mL or more achieved at least a PASI 90 response. [29]
Assessments of dosage pattern
Chaudhari et al. [17] concluded that PASI 75 was achieved at week 10 by 73% of patients dosed at 10 mg/kg in the induction phase compared to 82% of those dosed at 5 mg/kg. Gottlieb et al. [18] found the dose of 5 mg/kg to be better than 3 mg/kg for the induction phase. In a 16-week dose escalation trial to investigate withdrawal and retreatment with 10 mg/kg and 5 mg/kg of infliximab, patients who responded to treatment were withdrawn and monitored for relapse (defined as loss of at least half of the improvement in PASI at week 10). Patients who relapsed were retreated with a single-dose infusion of their original dose. This study reported that patients in the 5 mg/kg group sustained response up to 8 weeks of drug discontinuation compared to 12 weeks for the 10 mg/kg group. [18]
Menter et al. [27] compared the efficacy and safety of continuous (every 8 weeks) and intermittent (as needed) maintenance regimens in 835 patients. One group was initiated on 3 mg/kg, another, on 5 mg/kg, and the third, on placebo. After week 14 the infliximab group was assigned either the continuous regimen or the intermittent one (as clinically needed). At 50 weeks a better response was seen with continuous dosing than with intermittent dosing in both the 3 mg/kg and 5 mg/kg groups. Thus a continuous dosing pattern seems to be more beneficial than an intermittent dosing one.
In a study by Gottlieb et al. [30] infliximab was administered to all patients in three doses of 3 or 5 mg/kg as induction and then discontinued for 20 weeks. At week 10, 71.9% of patients on 3 mg/kg had PASI 75 compared to 87.9% of patients on 5 mg/kg. At week 26, 114 of 198 patients were administered a single additional infusion of infliximab; 38% of patients retreated with 3 mg/kg had clearance of lesions, compared to 64% of patients with 5 mg/kg at week 30.
In the study by Menter et al. [18] relapse was reported in majority patients between week 14-22 intermittent arm (defined by loss of PASI 75) although data were not shown. A small study indicated that 50% (15/30) patients relapse (loss of PASI 75) by week 26. There are no published prospective trial data beyond 1 year.
In the maintenance phase with an evaluation after 50 weeks, the percentage of patients maintaining a PASI 75 response decreased to 61% from 80% as studied by Reich et al. [19]
Guidelines from the American Academy of Dermatology (AAD), British Association of Dermatologists (BAD) and the International Consensus Panel of Dermatology experts advises dosing infliximab in a 5 mg/kg infusion schedule at 0, 2, and 6weeks; followed by maintenance treatment every 6-8 weeks [Table - 3]. [15],[21],[22],[24] The BAD guidelines, however, state that no studies have been performed to establish the optimal dose or frequency of repeated infusions required in order to achieve disease control. [15],[22] There are no clear recommendations on how to manage an attenuated response to infliximab.
Asian experience
There are few Asian studies of infliximab. In Japan, two clinical trials have been performed as phase III clinical trials: a randomized, double-blind, placebo-controlled multicenter clinical trial in patients with plaque psoriasis and psoriatic arthritis; and an open-label, uncontrolled multicenter trial in patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis, and psoriatic erythroderma. [29],[31] In the first trial, PASI 75 response was 68.6% at week 10, compared to 0 in placebo group. The PASI 75 and PASI 90 response rates in the infliximab group were 76.7% and 56.7%, respectively, at week 66.
A study by Torii et al., [32] on 90 Japanese patients reported a rapid improvement in the PASI score and sustained response to infliximab. The PASI 75, PASI 90, and PASI 100 response rates at the complete assessment at week 66 were 66.7%, 46.7%, and 17.8%, respectively. At the end of the trial, 90% or more of skin lesions in the head region disappeared in approximately 50% of the patients, with complete disappearance in approximately 41%. High PASI 75 and PASI 90 responses may be achieved irrespective of the body area. [32]
In an open label pilot Indian study, Sridhar et al. [33] treated three patients having moderate to severe plaque psoriasis with infliximab administered in a dose of 5 mg/kg and repeated at weeks 2 and 6. The study had two phases: A 10-week active phase, followed by a 12-week surveillance phase for relapse. During the active phase, infliximab was administered in a dose of 5 mg/kg and repeated at weeks 2 and 6. PASI was assessed at week 10. During the surveillance phase, PASI assessments were carried out at 4-week intervals until week 22 for relapse. All patients attained PASI 50 by 3.8 weeks and PASI 75 at 9.6 weeks. The mean improvement in PASI at week 10 was 77.2%. Relapse was reported at 18.6 weeks after the first infusion.
In the author′s experience, most patients with moderate to severe psoriasis show a response by week 8 to 10 with an induction dose of 5 mg/kg and relapse is seen on the legs and feet about 8 weeks after the induction phase. Articular symptoms however improve within a week of the first infusion. [34]
Efficacy and safety of infliximab vs methotrexate
In the Restore0 1 trial, MTX-naive patients (N = 868) were randomized 3:1 to receive infliximab 5 mg /kg at weeks 0, 2, 6, 14, and 22 or MTX 15 mg weekly with a dose increase to 20 mg weekly at week 6 if PASI response was < 25%. At week 16, patients with < PASI 50 response could switch treatment groups. The primary efficacy endpoint was PASI 75 response at week 16. Major secondary efficacy endpoints were PASI 75 response at week 26. Upon conclusion of this trial, the primary end point was more likely to be achieved with infliximab than with methotrexate (78 vs 42% of patients). The incidence of adverse effects was also higher in the infliximab treated group. Infliximab was well tolerated and more efficacious than MTX in patients with moderate-to-severe plaque psoriasis. Infliximab was also efficacious in patients who failed MTX and switched to infliximab. [35] A review by the Canadian Agency for Drugs and Technologies in Health (CADTH), based on meta-analysis of placebo-controlled trials and one RCT, noted that infliximab was more effective than methotrexate, etanercept, adalimumab and ustekinumab for the treatment of plaque psoriasis in adults. [36] However, according to limited data on safety, infliximab was more likely than methotrexate or 50 mg etanercept to be associated with adverse events.
Infliximab with concomitant methotrexate
Being a human chimeric monoclonal antibody, infliximab can incite neutralizing antibodies, which can be responsible for loss of efficacy with subsequent infusions in 27-47% of patients. These antidrug antibodies correlate with decreased serum infliximab levels and thus higher doses in subsequent infusions are needed. [37] Infusion reactions are 2-3 times more likely in patients who develop antibodies. The concomitant use of methotrexate may prevent the development of these antibodies and may reduce the frequency of infusion reactions. [38] The BAD guidelines state that concomitant systemic therapies may be indicated for some patients with very severe or unstable psoriasis. Improved efficacy with concomitant use of methotrexate is due to higher serum levels of infliximab and hence a recommendation to use smaller doses. [15] The AAD does not recommend concomitant prescription of low-dose methotrexate, although some clinicians do so to decrease the formation of antibodies. [24] The international consensus statement on the treatment of psoriasis with infliximab does not provide guidelines on the use of concomitant medication and infliximab. [15],[21]
Erythrodermic psoriasis
Erythrodermic psoriasis responds well and rapidly to infliximab, with clinical improvement being seen by the second infusion. [34],[39],[40],[41] According to a task force of the National Psoriasis Foundation, infliximab appears to be the most rapidly acting agent for the treatment of erythrodermic psoriasis and is recommended as a first line agent in the absence of contraindications. [42] Takahashi et al. [40] have reported that infliximab in combination with methotrexate is a promising choice for erythrodermic psoriasis. An excellent outcome at week 6, after third infusion in 7 patients with erythrodermic psoriasis was seen by treating the patients with infliximab 5 mg/kg at week 0, 2, 6 and 15 mg/week methotrexate. Heikkilδ et al. [41] reported similar improvement with infliximab (2.7-4.4 mg/kg) and methotrexate (5-7.5 mg/week). The combination with methotrexate reduces the risk of antichimeric antibodies. Infliximab was also successfully combined along with acitretin 0.3-0.6 mg/kg in a study by Takahashi et al. [40] on 7 patients with erythrodermic psoriasis and reported no adverse effects and good efficacy. The efficacy may be prolonged after induction when systemic therapies such as methotrexate or acitretin are combined, but long term follow-up data are scant. [40],[41] In a multicentre Japanese study, patients of erythrodermic psoriasis were assessed after infliximab at week 50 and week 66. PASI 90 responders in psoriatic erythroderma group were 37.5%. [32]
Pustular psoriasis
The most severe variety of psoriasis is the pustular type with the generalized pustular psoriasis having severe systemic manifestations of inflammation like fever, leucocytosis, joint pains, and often intractable to standard therapies. There are several reports of the rapid and sustained efficacy of infliximab in severe pustular psoriasis, [43],[44],[45],[46],[47] but most include only small numbers of patients and follow-up data is limited. [48] Infliximab down-regulates disease-promoting chemokines such as IL-8, growth regulated oncogene-alpha (GRO–α) and monocyte chemoattractant protein-1 (MCP-1) in pustular psoriasis. [49] It is usually administered every 6 weeks in a dose of 5 mg/kg. Routhouska et al. [50] reported success with the addition of weekly, low dose methotrexate. This is echoed by the author′s experience of two generalized pustular psoriaisis patients who showed remarkable clinical improvement within 48 h of the first infusion and concomitant methotrexate 5 mg/week. No relapse was seen after the induction phase in two of the patients for 3 years. [34],[51]
Nail psoriasis
Nail psoriasis runs a protracted course and is difficult to treat. Although its response to infliximab was slower than that of skin plaques in a Japanese study, substantial improvement was seen with long-term therapy, even in severe cases involving all 10 finger nails. [32] Furthermore, the improvement in NAPSI (Nail Psoriasis Severity Index) score was evident in the nail matrix as well as in the nail bed, and approximately 50% of patients achieved complete clearance. In another study, over 40% of 373 infliximab treated patients with moderate to severe plaque psoriasis showed nail clearance at 50 weeks. [52] Significant NAPSI improvement at week 38 was reported in 46 patients with nail psoriasis after 6 infliximab infusions. [53] In a small series, all 25 patients who received infusions of infliximab 5 mg/kg had clinical remission, with a NAPSI 75 by week 22, which was maintained at a follow up 12 weeks after the final infusion. [54]
Worsening of psoriasis after infliximab
Paradoxical activation of psoriasis, including pustular psoriasis and palmoplantar pustulosis, with infliximab treatment has been occasionally observed in adults and in children. [55],[56],[57],[58],[59],[60] Mössner et al. [57] reported 5 patients with chronic plaque-type psoriasis who developed palmoplantar pustulosis during or after discontinuation of infliximab therapy. In two of the five cases, manifestation of palmoplantar pustulosis was not accompanied by worsening of plaque-type psoriasis. This study postulates that site-specific factors or a differential contribution of immunological processes modulated by TNF-α inhibitors to palmoplantar pustulosis and plaque-type psoriasis may be responsible for this expression.
Wollina et al. [58] reviewed TNF-α antagonist induced psoriasis or psoriasiform eruptions in 120 patients, including 63 on infliximab. In 74 patients, the psoriasis was newly diagnosed and in 25, there was exacerbation or aggravation of pre-existing psoriasis. Withdrawal of the implicated TNF-α inhibitor in 47 patients alone or in association with adjuvant anti-psoriatic topical therapy resulted in complete remission in 21 patients, partial remission in 20, and stable disease in another three. Patients who develop psoriasis or a psoriasiform eruption during treatment with one TNF-α blocker may respond to an alternative TNF-α antagonist without any adverse event. [59],[60] In children, infliximab induced psoriasis may be present with involvement of the scalp [61] or face. [55]
Pediatric psoriasis
Infliximab has been used for Crohn′s disease in children older than 6 years. Though there are no trials of its use for psoriasis in children, there are several reports of its successful use in psoriasis. Three cases have been reported, all the treated children were above 8 years of age. Two of chronic plaque recalcitrant psoriasis and another of generalized pustular psoriasis. Two patients were treated with induction dose 3mg/kg and one with 5 mg/kg and maintenance at intervals 8 weeks. Improvement was reported in all the cases. Recurrence was seen on scalp and limbs after 10 months in the patient with pustular psoriasis and was controlled with addition of methotrexate. [61],[62],[63],[64]
Pregnancy and lactation
According to European guidelines, infliximab is not recommended during pregnancy or breastfeeding (FDA pregnancy category B). Because of the long half-life of the product, reliable contraception is required in women of childbearing potential until 6 months after the last infusion. [23]
In BAD guidelines infliximab is not recommended in pregnancy and lactation. There are no prospective or retrospective trials that been conducted for the treatment of psoriasis during pregnancy with infliximab. Several publications concerning the outcome of pregnancy following exposure to TNF antagonists are reported in Crohn′s disease and arthritis. Although these patients, in contrast to patients with psoriasis, are more likely to have been exposed to combination therapy. [15]
Lactation safety is unknown according to various guidelines. [15],[21],[22],[24]
Conclusion
Infliximab is the biologic therapeutic option for patients with moderate to severe psoriasis that is non-responsive to conventional antipsoriatic agents. Infliximab is extremely effective and has a rapid onset of action. The response is usually evident by the second week of treatment. There are little data related to the use of infliximab for moderate to severe psoriasis in Indian patients, but anecdotal reports indicate its efficacy and rapid onset of action. International guidelines recommend standard dose used is 5 mg/kg body weight. Standard induction is of 3 doses. Choice between intermittent or continuous dosing pattern is dependent on clinical response and maintenance of response, taking into consideration the safety issues and prevention of potential antichimeric antibody development. Infliximab is an excellent option for generalized pustular and erythrodermic psoriasis that are recalcitrant to standard anti-psoriatic therapy. A vial of 100mg costs 34,000 INR. For an average Indian weighing 70 kg, one induction dose of 5 mg/kg is approximately 136,000 INR. In India prospective studies are needed to establish the efficacy and safety of biologics in recalcitrant moderate to severe plaque psoriasis.
| 1. |
Mehlis SL, Gordon KB. The Immunology of psoriasis and biologic immunotherapy. J Am Acad Dermatol 2003;49:S44-50.
[Google Scholar]
|
| 2. |
Gordon KB, West DP. Biologic therapy in dermatology. In: Wolverton SE, editor. Comprehensive Dermatologic Drug Therapy. 2 nd ed. Philadelphia: WB Saunders. 2002. p. 928-42.
[Google Scholar]
|
| 3. |
Asadullah K, Sterry W, Trefzer U. Cytokines: Interleukin and interferon therapy in dermatology. Clin Exp Dermatol 2002;27:578-84.
[Google Scholar]
|
| 4. |
O′Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol 2002;2:37-45.
[Google Scholar]
|
| 5. |
Gottlieb AB. Clinical research helps elucidate the role of tumor necrosis factor-alpha (TNF-alpha) in the pathogenesis of T1-mediated immune disorders: Use of targeted immunotherapeutics as pathogenic probes. Lupus 2003;12:190-4.
[Google Scholar]
|
| 6. |
Onuma S. Immunohistochemical studies of infiltrating cells in early and chronic lesions of psoriasis. J Dermatol 1994;21:223-32.
[Google Scholar]
|
| 7. |
Oh CJ, Das KM, Gottlieb AB. Treatment with anti-tumor necrosis factor-alpha (TNF-alpha) monoclonal antibody dramatically decreases the clinical activity of psoriasis lesions. J Am Acad Dermatol 2000;42:829-30.
[Google Scholar]
|
| 8. |
Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med 2004;199:125-30.
[Google Scholar]
|
| 9. |
Mussi A, Bonifati C, Carducci M, D′Agosto G, Pimpinelli F, D′Urso D, et al. Serum TNF- α level correlate with disease severity and are reduced by effective therapy in plaque-type Psoriasis. J Biol Regul Homeost Agents 1997;11:115-8.
[Google Scholar]
|
| 10. |
Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol 2002;46:1-23.
[Google Scholar]
|
| 11. |
Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNF-alpha monoclonal anti-body cA2 binds recombinant transmembrane TNF-alpha and activates immune effector functions. Cytokine 1995;7:251-9.
[Google Scholar]
|
| 12. |
Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol Ther 2008;117:244-79.
[Google Scholar]
|
| 13. |
ten Hove T, van Montfrans C, Peppelenbosch MP, van Deventer SJ. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn′s disease. Gut 2002;50:206-11.
[Google Scholar]
|
| 14. |
Mössner R, Schön MP, Reich K. Tumor necrosis factor antagonists in the therapy of psoriasis. Clin Dermatol 2008;26:486-502.
[Google Scholar]
|
| 15. |
Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler DA, et al. British Association of Dermatologists′ guidelines for biologic interventions for psoriasis 2009. Br J Dermatol 2009;161:987-1019.
[Google Scholar]
|
| 16. |
Atzeni F, Sarzi-Puttini P, Doria A, Iaccarino L, Capsoni F. Potential off-label use of infliximab in autoimmune and non-autoimmune diseases: A review. Autoimmun Rev 2005;4:144-52.
[Google Scholar]
|
| 17. |
Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: A randomized trial. Lancet 2001;357:1842-7.
[Google Scholar]
|
| 18. |
Gottlieb AB, Chaudhari U, Mulcahy LD, Li S, Dooley LT, Baker DG. Infliximab monotherapy provides rapid and sustained benefit for plaque type psoriasis. J Am Acad Dermatol 2003;48:829-35.
[Google Scholar]
|
| 19. |
Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: A phase III, multicentre, double-blind trial. Lancet 2005;366:1367-74.
[Google Scholar]
|
| 20. |
de Vries HS, van Oijen MG, Driessen RJ, de Jong EM, Creemers MC, Kievit W, et al. Appropriate infliximab infusion dosage and monitoring: Results of a panel meeting of rheumatologists, dermatologists and gastroenterologists. Br J Clin Pharmacol 2011;71:7-19.
[Google Scholar]
|
| 21. |
Reich K, Griffiths C, Barker J, Chimenti S, Daudén E, Giannetti A, et al. Recommendations for the long-term treatment of psoriasis with infliximab: A dermatology expert group consensus. Dermatology 2008;217:268-75.
[Google Scholar]
|
| 22. |
Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler D, et al. British Association of Dermatologists guidelines for use of biological interventions in psoriasis 2005. Br J Dermatol 2005;153:486-97.
[Google Scholar]
|
| 23. |
Pathirana D, Ormerod AD, Saiag P, Smith C, Spuls PI, Nast A, et al. European S3-Guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol 2009;23:1-70.
[Google Scholar]
|
| 24. |
Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008;58:826-50.
[Google Scholar]
|
| 25. |
Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol 2005;152:861-7.
[Google Scholar]
|
| 26. |
Feldman SR, Gordon KB, Bala M, Evans R, Li S, Dooley LT, et al. Infliximab treatment results in significant improvement in the quality of life of patients with severe psoriasis: A double-blind placebo-controlled trial. Br J Dermatol 2005;152:954-60.
[Google Scholar]
|
| 27. |
Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2007;56:31.e1-15.
[Google Scholar]
|
| 28. |
Vincek V, Jacob SE, Nassiri M, Herbert LM, Nadji M, Kerdel FA. Infliximab Monotherapy in psoriasis: A case of rapid clinical and histological response. Int J Dermatol 2004;43:303-8.
[Google Scholar]
|
| 29. |
Torii H, Nakagawa H. Japanese Infliximab Study Investigators. Long term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol 2011;38:321-34.
[Google Scholar]
|
| 30. |
Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: A randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol 2004;51:534-42.
[Google Scholar]
|
| 31. |
Torii H, Nakagawa H. Japanese Infliximab Study investigators. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci 2010;59:40-9.
[Google Scholar]
|
| 32. |
Torii H, Sato N, Yoshinari T, Nakagawa H. Japanese Infliximab Study Investigators. Dramatic impact of a Psoriasis Area and Severity Index 90 response on the quality of life in patients with psoriasis: An analysis of Japanese clinical trials of infliximab. J Dermatol 2012;39:253-9.
[Google Scholar]
|
| 33. |
Sridhar J, Desylva P, Singh YD. Chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) in psoriasis. Indian J Dermatol Venereol Leprol 2006;72:133-5.
[Google Scholar]
|
| 34. |
Arsiwala S. Biologics. In: Shroff HJ, editor. Psoriasis Optimizing Therapeutic Response. 1 st ed. Mumbai: Bhalani Publishers; 2010. p. 73-86.
[Google Scholar]
|
| 35. |
Barker J, Hoffmann M, Wozel G, Ortonne JP, Zheng H, van Hoogstraten H, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: Results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol 2011;165:1109-17.
[Google Scholar]
|
| 36. |
Infliximab versus methotrexate, etanercept, adalimumab, and ustekinumab for plaque psoriasis. CADTH Rapid Response Report: Canadian Agency for Drugs and Technologies in Health (CADTH). Available from: http://www.cadth.ca/media/pdf//htis/aug-012/RC0369%20Infliximab%20Final.pdf. [Last cited on 2012 Aug 10].
[Google Scholar]
|
| 37. |
Rott S, Mrowietz U. Recent developments in the use of biologics in psoriasis and autoimmune disorders. The role of autoantibodies. BMJ 2005;330:716-20.
[Google Scholar]
|
| 38. |
Thappa DM, Laxmisha C. Immunomodulators in the treatment of psoriasis. Indian J Dermatol Venereol Leprol 2004;70:1-9.
[Google Scholar]
|
| 39. |
Poulalhon N, Begon E, Lebbé C, Lioté F, Lahfa M, Bengoufa D, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: Evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol 2007;156:329-36.
[Google Scholar]
|
| 40. |
Takahashi MD, Castro LG, Romiti R. Infliximab, as sole or combined therapy, induces rapid clearing of erythrodermic psoriasis. Br J Dermatol 2007;157:828-31.
[Google Scholar]
|
| 41. |
Heikkilä H, Ranki A, Cajanus S, Karvonen SL. Infliximab combined with methotrexate as long-term treatment for erythrodermic psoriasis. Arch Dermatol 2005;141:1607-10.
[Google Scholar]
|
| 42. |
Rosenbach M, Hsu S, Korman NJ, Lebwohl MG, Young M, Bebo BF Jr, et al. Treatment of erythrodermic psoriasis: From the medical board of the National Psoriasis Foundation. J Am Acad Dermatol 2010;62:655-62.
[Google Scholar]
|
| 43. |
Trent JT, Kerdel FA. Successful treatment of Von Zumbush pustular psoriasis with infliximab. J Cutan Med Surg 2004;8:224-8.
[Google Scholar]
|
| 44. |
Schmick K, Grabbe J. Recalcitrant, generalized pustular psoriasis: Rapid and lasting therapeutic response to antitumour necrosis factor-alpha antibody (infliximab). Br J Dermatol 2004;150:367.
[Google Scholar]
|
| 45. |
Lewis TG, Tuchinda C, Lim HW, Wong HK. Life-threatening pustular and erythrodermic psoriasis responding to infliximab. J Drugs Dermatol 2006;5:546-8.
[Google Scholar]
|
| 46. |
Weisenseel P, Prinz JC. Sequential use of infliximab and etanercept in generalized pustular psoriasis. Cutis 2006;78:197-9.
[Google Scholar]
|
| 47. |
Vieira Serrão V, Martins A, Lopes MJ. Infliximab in recalcitrant generalized pustular arthropatic psoriasis. Eur J Dermatol 2008;18:71-3.
[Google Scholar]
|
| 48. |
Newland MR, Weinstein A, Kerdel F. Rapid response to infliximab in severe pustular psoriasis, von Zumbusch type. Int J Dermatol 2002;41:449-52.
[Google Scholar]
|
| 49. |
Benoit S, Toksoy A, Bröcker EB, Gillitzer R, Goebeler M. Treatment of recalcitrant pustular psoriasis with infliximab: Effective reduction of chemokine expression. Br J Dermatol 2004;150:1009-12.
[Google Scholar]
|
| 50. |
Routhouska SB, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: Case series. J Cutan Med Surg 2008;12:184-8.
[Google Scholar]
|
| 51. |
Arsiwala SZ. Rapid and sustained response of acute generalized pustular psoriasis to infliximab. In: Shroff HJ, editor. Casebook of Dermatology. vol. 4. New Delhi: IJCP Publishers; 2012. p. 35-9.
[Google Scholar]
|
| 52. |
Rich P, Griffiths CE, Reich K, Nestle FO, Scher RK, Li S, et al. Baseline nail disease in patients with moderate to severe psoriasis and response to treatment with infliximab during 1 year. J Am Acad Dermatol 2008;58:224-31.
[Google Scholar]
|
| 53. |
Rigopoulos D, Gregoriou S, Stratigos A, Larios G, Korfitis C, Papaioannou D, et al. Evaluation of the efficacy and safety of infliximab on psoriatic nails: An unblinded, non randomized, open label study. Br J Dermatol 2008;159:453-6.
[Google Scholar]
|
| 54. |
Bianchi L, Bergamin A, de Felice C, Capriotti E, Chimenti S. Remission and time of resolution of nail psoriasis during infliximab therapy. J Am Acad Dermatol 2005;52:736-7.
[Google Scholar]
|
| 55. |
Hiremath G, Duffy L, Leibowitz I. Infliximab-induced psoriasis in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2011;52:230-2.
[Google Scholar]
|
| 56. |
Martínez-Morán C, Sanz-Muñoz C, Morales-Callaghan AM, Torrero V, Miranda-Romero A. Pustular psoriasis induced by infliximab. J Eur Acad Dermatol Venereol 2007;21:1424-6.
[Google Scholar]
|
| 57. |
Mössner R, Thaci D, Mohr J, Pätzold S, Bertsch HP, Krüger U, et al. Manifestation of palmoplantar pustulosis during or after infliximab therapy for plaque-type psoriasis: Report on five cases. Arch Dermatol Res 2008;300:101-5.
[Google Scholar]
|
| 58. |
Wollina U, Hansel G, Koch A, Schönlebe J, Köstler E, Haroske G. Tumor necrosis factor-alpha inhibitor-induced psoriasis or psoriasiform exanthemata: First 120 cases from the literature including a series of six new patients. Am J Clin Dermatol 2008;9:1-14.
[Google Scholar]
|
| 59. |
Collamer AN, Guerrero KT, Henning JS, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: A literature review and potential mechanisms of action. Arthritis Rheum 2008;59:996-1001.
[Google Scholar]
|
| 60. |
Satriano RA, Abbate G, Esposito S, Cassaglia B, Piccolo V, Baroni A. "Paradoxical" adverse effects caused by anti-tumor necrosis factor-a biological drugs: Appearance of psoriasis in a patient treated with infliximab for rheumatoid arthritis. Indian J Dermatol Venereol Leprol 2011;77:536.
[Google Scholar]
|
| 61. |
Perman MJ, Lovell DJ, Denson LA, Farrell MK, Lucky AW. Five cases of anti-tumor necrosis factor alpha-induced psoriasis presenting with severe scalp involvement in children. Pediatr Dermatol 2012;29:454-9.
[Google Scholar]
|
| 62. |
Menter MA, Cush JM. Successful treatment of pediatric psoriasis with Infliximab. Pediatr Dermatol 2004;21:87-8.
[Google Scholar]
|
| 63. |
Pereira TM, Vieira AP, Fernandes JC, Antunes H, Basto AS. Anti-TNF alpha therapy in childhood pustular psoriasis. Dermatology 2006;213:350-2.
[Google Scholar]
|
| 64. |
Rott S, Küster RM, Mrowietz U. Successful treatment of severe psoriatic arthritis with infliximab in an 11-year-old child suffering from linear psoriasis along lines of Blaschko. Br J Dermatol 2007;157:191-2.
[Google Scholar]
|
Fulltext Views
7,931
PDF downloads
2,393





