Translate this page into:
Is there a correlation of serum and tissue T helper-1 and -2 cytokine profiles with psoriasis activity and severity? A cross-sectional study
2 Department of Biochemistry, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Sujay Khandpur
Department of Dermatology and Venereology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi - 110 029
India
| How to cite this article: Khandpur S, Gupta V, Das D, Sharma A. Is there a correlation of serum and tissue T helper-1 and -2 cytokine profiles with psoriasis activity and severity? A cross-sectional study. Indian J Dermatol Venereol Leprol 2018;84:414-418 |
Abstract
Background: Previous studies correlating Th1 and Th2 cytokine profiles with psoriasis activity provided inconsistent results. Correlation of tissue cytokine levels with psoriasis severity has not been studied till now.
Objective: To compare serum and tissue Th1 and Th2 cytokine profiles of patients with active and stable psoriasis as well as healthy controls, and to correlate them with psoriasis severity.
Methodology: This was a cross-sectional study involving adult patients with 'active' psoriasis (untreated progressive chronic plaque psoriasis, guttate psoriasis, and erythrodermic psoriasis), 'stable' psoriasis (stable plaque psoriasis or those with completely resolved lesions) and healthy subjects with non-inflammatory skin lesions as controls. Mean levels of Th1 and Th2 cytokines in serum [interleukin 2 (IL-2), interferon-gamma (IFN-γ), IL-4, IL-10] and tissue mRNA expression (IFN-γ, IL-4) were compared among these three groups.
Results: There were 30 patients each in active and stable psoriasis groups, and 15 in the control group. Mean serum IL-2, IFN-γ, and IL-10 levels of patients with psoriasis patients were significantly higher than the controls (P < 0.001 for both active and stable psoriasis), whereas mean serum IL-4 level of patients was significantly lower than the controls (P < 0.001). However, there was no statistically significant difference of serum cytokine levels between active and stable psoriasis groups. Mean quantitative tissue mRNA expression of IFN-γ and IL-4 of patients with active and stable psoriasis were significantly lower than the controls (P < 0.001 and <0.01, respectively), but were not significantly different between active and stable psoriasis groups. Serum and tissue cytokines showed weak correlation with psoriasis area and severity index.
Limitations: Small sample size and heterogenous nature of patients with psoriasis in terms of disease activity, morphology and treatment are limitations of this study.
Conclusions: There is no significant change in the serum or tissue levels of Th1 and Th2 cytokines with activity or severity of psoriasis.
Introduction
Imbalance between T-helper 1 (Th1) and T-helper (Th2) cytokines in psoriasis is well-recognized. Th1 cytokines are considered to be upregulated, whereas Th2 cytokines are downregulated. Previous studies have reported that serum Th1 cytokines are elevated in patients with psoriasis compared to controls, and it correlated positively with severity of psoriasis.[1],[2],[3],[4] Surprisingly, some researchers have reported that serum IL-4 (a Th2 cytokine) levels are elevated among patients with psoriasis compared to controls.[5],[6] Studies comparing serum or tissue cytokine profiles in psoriasis before and after treatment have not yielded consistent results.[5],[7],[8] Correlation of tissue cytokine levels with severity of psoriasis has not been studied till date. The present study aims to compare serum and tissue Th1 and Th2 cytokine profiles of patients with active and stable psoriasis and healthy controls, and to correlate them with severity of psoriasis.
Methodology
This was a cross-sectional study conducted in the department of dermatology and venereology of All India Institute of Medical Sciences, New Delhi, India over a period of one year (September 2014 to August 2015) after approval from the Institute Ethics Committee.
Study participants
Patients with psoriasis: Adult patients (≥18 years) with guttate psoriasis, progressive lesions of chronic plaque psoriasis or erythrodermic psoriasis, not on systemic antipsoriatic medication (modern medical or indigenous) during the previous four weeks, and not on topical therapy during the previous two weeks were recruited to the “active untreated psoriasis” group after obtaining informed consent. Patients with chronic plaque psoriasis, with no progression of lesions for at least four weeks or those with currently subsided lesions were included in the “stable psoriasis” group. Patients with pustular psoriasis, psoriatic arthropathy or co-existing systemic or cutaneous illnesses were excluded. History and examination findings were recorded in a predesigned proforma. Severity of psoriasis was assessed using psoriasis area severity index (PASI) score.
Controls: Age and gender-matched, apparently healthy subjects with non-inflammatory dermatoses such as nevi, lipomas, scars, etc., were recruited as controls after obtaining informed consent.
Estimation of serum Th1 and Th2 cytokine levels by enzyme-linked immunosorbent assay
Commercially available enzyme-linked immunosorbent assay (ELISA) kits (BosterBio, USA) were used for estimating serum levels of Th1 [interleukin 2 (IL-2), interferon-gamma (IFN-γ)] and Th2-associated cytokines (IL-4, IL-10). Venous blood samples obtained from patients and controls were diluted and measured in triplicate. The reaction was terminated by adding sulfuric acid and absorbance was measured at 450 nm. The absorbance measured by ELISA reader was proportional to the concentration of respective cytokine present in the sample.
Quantitative mRNA expression of IFN-γ and IL-4 cytokines in tissue by real-time polymerase chain reaction
Skin tissue (4 mm punch skin biopsy) dissolved in TRIZOL reagent in a 1.5 ml tube was used for extraction of total RNA. It was incubated at room temperature (18–25°C) for 10 min. Chloroform 200 μl was added and vortexed for 15 s followed by incubation at room temperature for 2–3 min. The samples were then centrifuged at 12,000g for 15 min at 40°C. Following centrifugation, three phases become visible in the tube. The upper aqueous phase containing RNA was carefully transferred to a fresh tube, without allowing it to mix with other phases. 500 μl of isopropanol was added to the tube and incubated at room temperature for 10 min. The sample was centrifuged at 12,000g for 10 min at 40°C. The supernatant was carefully removed after centrifugation, without disturbing the pellet. The RNA pellet so obtained was washed with 70% ethyl alcohol and centrifuged at 7500g for 8 min at 40°C. The remaining ethyl alcohol was allowed to air dry for 2–3 min. The pellet was re-dissolved in 30 μl of diethyl pyrocarbonate water. The DNAse treated RNA so obtained from the tissue was used to synthesize complementary DNA (cDNA) using MuLV reverse transcriptase (Fermentas), used as template to analyze the amplification using primers specific to the different cytokines (Th1: IFN-γ, Th2: IL-4). The primers were procured from Integrated DNA Technologies (IDT, USA). The cDNA so obtained was diluted and used as template for quantitative polymerase chain reaction (qPCR). Maxima SYBR Green qPCR Master Mix (2X) (Fermentas) was used to perform the relative mRNA expression analysis, calculated by 2−ΔCt method. Primers were standardized using gradient PCR with a range of temperatures (55–65°C) to carry out molecular expression studies. Final forward and reverse primers chosen for IFN-γ were 5′GAGTGTGGAGACCATCAAGGAAG3′ and 5′CAGTTCAGCC ATCACTTGGAT3′ respectively, whereas forward and reverse primers for IL-4 were 5′CACCATGAGAAGGACACTCG3′ and 5′CGTACTCTGGTTG GCTTCCT3′ respectively. Final temperature used for qPCR was 60°C.
The study flowchart is depicted in [Figure - 1].
 |
| Figure 1: The study flowchart |
Statistical analysis
Tissue and serum cytokine levels of patients with active and stable psoriasis, and controls were compared using Wilcoxon rank-sum test. Correlation between serum and tissue cytokine levels, and between cytokine levels (serum and tissue) and PASI were estimated using Spearman's rank correlation coefficient. P value ≤0.05 was considered significant. Statistical analysis was performed using Stata 12 software (College Station, TX: StataCorp LP. 2011).
Results
There were 30 patients each in active and stable psoriasis groups, and 15 in the control group. Demographic profile and baseline characteristics of patients and controls are summarized in [Table - 1].
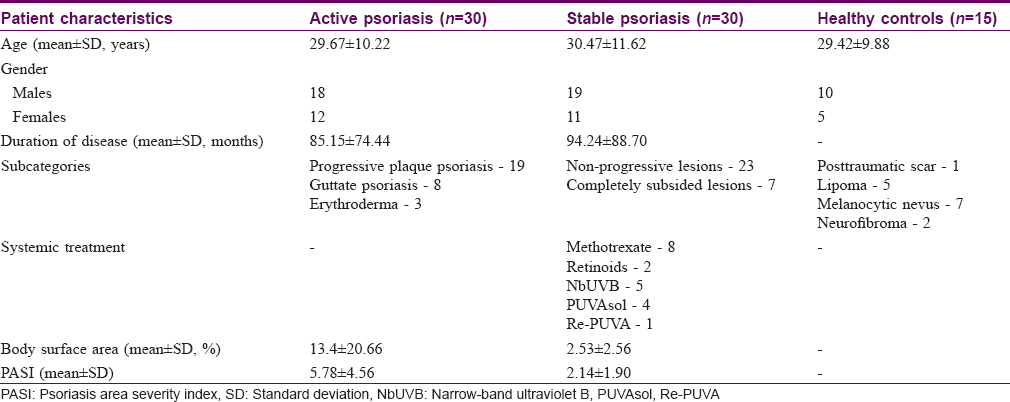
Comparison of serum and tissue cytokine levels among patients with active psoriasis, stable psoriasis and controls
Mean serum IL-2, IFN-γ, IL-4 and IL-10 levels and tissue IFN-γ and IL-4 cytokine mRNA expression levels among patients and controls are summarized in [Table - 2]. Mean serum IL-2, IFN-γ, and IL-10 levels among patients with both active and stable psoriasis were significantly higher than controls (P< 0.001 for both the groups), whereas mean serum IL-4 level was significantly lower in patients with psoriasis than controls (P< 0.001 for both the groups). There was no statistically significant difference in any of the serum cytokine mean levels between active and stable psoriasis groups.
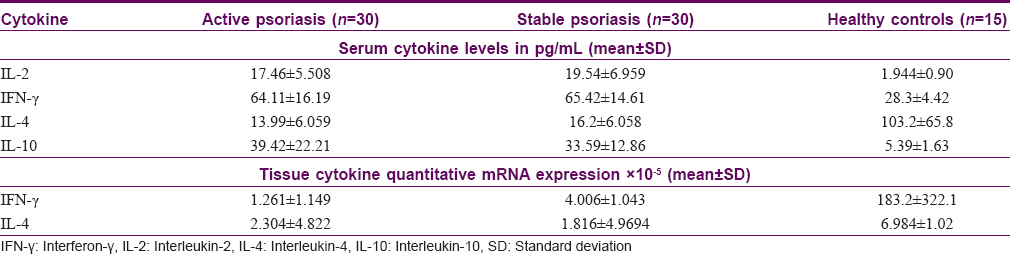
Mean quantitative mRNA expression levels of both IFN-γ and IL-4 in the lesional skin of patients with active and stable psoriasis were significantly lower than the controls (P< 0.001 and <0.01, respectively). Similar to serum cytokine analysis, there was no significant difference in either of these tissue cytokine mRNA expression levels between patients with active and stable psoriasis.
A post hoc subgroup analysis did not reveal statistically significant difference in mean serum or tissue cytokine levels between patients with progressive plaque psoriasis (n = 19) and those with guttate or erythrodermic psoriasis (n = 11); between patients with stable non-progressive psoriasis (n = 23) and those with completely subsided psoriatic lesions (n = 7); and between patients on systemic treatment (n = 20) and not on systemic treatment (n = 40), irrespective of disease activity.
Correlation of serum and tissue cytokine levels with psoriasis severity
Mean levels of all the serum and tissue cytokines, except serum IL-10, showed a weak negative correlation with psoriasis severity as assessed by PASI. For the purpose of subgroup analysis, PASI was categorized as ≤5 (n = 48), 6–10 (n = 7) and >10 (n = 5). None of the correlation analysis was statistically significant. The results of correlation analysis of cytokine levels with PASI are given in [Table - 3].
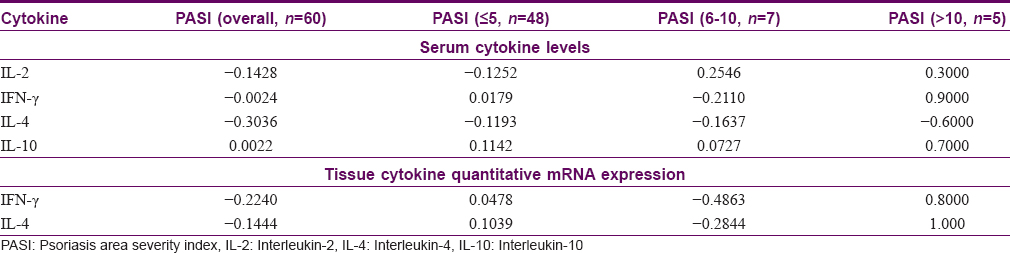
Correlation between serum and tissue cytokine levels
Mean serum IFN-γ and IL-4 levels showed a statistically insignificant, weak positive correlation with their corresponding mean tissue mRNA expression levels (r = 0.029 and 0.123, respectively). However, post hoc subgroup analysis showed reversal of this correlation for serum and tissue IFN-γ among patients with active psoriasis (IFN-γ, r = −0.0391; IL-4, r = 0.2753), and for serum and tissue IL-4 among patients with stable psoriasis (IFN-γ, r = 0.1102; IL-4, r = −0.0146).
Discussion
Our results which showed statistically significant higher serum levels of IL-2 and IFN-γ, and lower serum as well as tissue IL-4 levels among patients with psoriasis as compared to healthy controls are in agreement with majority of the previous studies.[1],[2],[3],[4],[9] Interestingly, higher IL-4 levels also have been reported among patients with psoriasis compared to controls.[5],[6] IL-4 may indirectly orchestrate an IFN-γ-mediated Th1 response by stimulating the development of IL-12 producing dendritic cells.[10]
There is divergence in the published literature regarding our finding of significantly raised serum IL-10 levels in psoriasis. Borska et al. found serum IL-10 levels to be elevated among patients with psoriasis.[11] These reduced following treatment with Goeckerman regimen. But, IL-10 was reported to be decreased in 122 patients with psoriasis compared to 78 healthy controls in a study from Japan.[4]
Though we found serum IFN-γ levels to be significantly higher among patients with psoriasis, our findings of lower tissue IFN-γ levels among them was unexpected. This is in contrast to some earlier studies.[9],[12],[13] There may be several reasons for this discordance. As IFN-γ signaling is multifaceted, some not yet known factor in its pathogenesis might have affected tissue levels of IFN-γ in our study population. Recently, it has been discovered that IFN-γ may have an unexpected paradoxical role in the control of auto-inflammation.[14],[15] It can act as a pro-inflammatory molecule as well as regulate immune response. Further, as we studied only the mRNA expression of IFN-γ, we cannot comment on the proteomics of IFN-γ signaling pathway and its regulatory molecules.
Given that psoriasis is predominantly a Th1-mediated disease with a simultaneous downregulation of Th2 axis, one would expect the imbalance between Th1 and Th2 cytokines to disappear with disease stability. However, previous studies evaluating changes in cytokine levels with disease activity have not provided consistent results. Moreover, most of these studies have evaluated serum cytokine levels, instead of tissue levels which may reflect disease activity better. Successful treatment with phototherapeutic modalities is accompanied by a reduction in serum IL-2, IFN-γ, IL-12, and IL-10 levels.[11],[16] Acitretin was reported to reduce serum and tissue levels of IFN-γ, but had no effect on IL-4 levels.[7] Etanercept was shown to produce a significant upregulation of IL-2 and downregulation of IL-12 levels in clinical responders.[5] Serum levels of IL-4 and IFN-γ also increased during treatment with etanercept, but this did not reach statistical significance.[5] On the contrary, treatment with ustekinumab failed to show any significant difference in the serum levels of inflammatory cytokines, including IFN-γ, IL-10, and IL-12 over a 12-week period.[17] It is possible that the change in Th1 and Th2 cytokine profiles is driven by the nature of therapeutic modality as much as by clinical improvement itself.
We did not find any significant difference between serum or tissue cytokine levels among patients with psoriasis based on disease activity, i.e. between active and stable psoriasis. This could probably be because all the patients in our study were not treated with the same modality. In fact, one-third of our patients with stable psoriasis were not on any systemic anti-psoriatic treatment.
In our study, only serum IL-10 exhibited a positive, though poor, correlation with PASI, whereas serum IL-2, IFN-γ, and IL-4 levels showed a weak negative correlation. Tissue IFN-γ and IL-4 levels also had a negative correlation with PASI. In contrast, some earlier studies have shown serum IFN-γ and IL-2 to correlate positively with psoriasis severity,[2],[3],[4] and serum IL-10 to correlate negatively.[4] Our results on correlation of cytokine levels with disease severity also are in contrast with earlier studies.[2],[3],[4] Subgroup analysis revealed that among patients with PASI ≥10, serum and tissue levels of all the cytokines, except serum IL-4, correlated positively with disease severity. However, our sample size was too small for the subgroup analysis to be meaningful.
We found a weak positive correlation between serum and tissue IFN-γ and IL-4 levels. The source of inflammatory cytokines in serum and tissue are different. These are secreted by innate immune cells and activated T-lymphocytes in serum whereas their tissue level depends on the migration of inflammatory cells to tissue-specific sites. The migratory potential of reactive immune cells may determine the correlation between tissue and serum cytokine levels. This needs further evaluation.
Our study is limited by a small sample size and the heterogeneity of activity, morphology and treatment taken for psoriasis. We tried to minimize the confounding factors by recruiting age and gender-matched subjects as controls. Care was taken to exclude those with systemic illnesses such as diabetes, hypertension, endocrinological abnormalities, malignancy, overt infections, chronic renal disease and medications, which could influence cytokine profile.
Conclusions
Our results support the role of upregulated Th1 cytokines in the pathogenesis of psoriasis. The present study shows that Th1 and Th2 cytokines do not change substantially with disease activity or severity. Future studies should investigate the role of cellular phenotypes, functional markers, chemokine receptors and ligands implicated in the pathogenesis of psoriasis, which may serve as novel therapeutic targets.
Acknowledgment
We would like to acknowledge the assistance of Mr. Vishwajeet Singh, PhD Scholar, Department of Biostatistics, AIIMS, New Delhi for his help in statistical analysis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. Patients have given their consent for their clinical information to be reported in the journal. Patients understand that their names and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
The study received intra-mural funding (Project no. A-122) from All India Institute of Medical Sciences, New Delhi, India.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Zhu K, Ye J, Wu M, Cheng H. Expression of Th1 and Th2 cytokine-associated transcription factors, T-bet and GATA-3, in peripheral blood mononuclear cells and skin lesions of patients with psoriasis vulgaris. Arch Dermatol Res 2010;302:517-23.
[Google Scholar]
|
| 2. |
Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm 2005;2005:273-9.
[Google Scholar]
|
| 3. |
Jacob SE, Nassiri M, Kerdel FA, Vincek V. Simultaneous measurement of multiple Th1 and Th2 serum cytokines in psoriasis and correlation with disease severity. Mediators Inflamm 2003;12:309-13.
[Google Scholar]
|
| 4. |
Takahashi H, Tsuji H, Hashimoto Y, Ishida-Yamamoto A, Iizuka H. Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin Exp Dermatol 2010;35:645-9.
[Google Scholar]
|
| 5. |
Quaglino P, Bergallo M, Ponti R, Barberio E, Cicchelli S, Buffa E, et al. Th1, Th2, Th17 and regulatory T cell pattern in psoriatic patients: Modulation of cytokines and gene targets induced by etanercept treatment and correlation with clinical response. Dermatology 2011;223:57-67.
[Google Scholar]
|
| 6. |
Zalewska A, Wyczółkowska J, Dziankowska-Bartkowiak B, Sysa-Jedrzejowska A. Interleukin 4 plasma levels in psoriasis vulgaris patients. Med Sci Monit 2004;10:CR156-62.
[Google Scholar]
|
| 7. |
Niu X, Cao W, Ma H, Feng J, Li X, Zhang X. Acitretin exerted a greater influence on T-helper (Th) 1 and Th17 than on Th2 cells in treatment of psoriasis vulgaris. J Dermatol 2012;39:916-21.
[Google Scholar]
|
| 8. |
Antiga E, Volpi W, Cardilicchia E, Maggi L, Filì L, Manuelli C, et al. Etanercept downregulates the Th17 pathway and decreases the IL-17+/IL-10+ cell ratio in patients with psoriasis vulgaris. J Clin Immunol 2012;32:1221-32.
[Google Scholar]
|
| 9. |
Coimbra S, Figueiredo A, Castro E, Rocha-Pereira P, Santos-Silva A. The roles of cells and cytokines in the pathogenesis of psoriasis. Int J Dermatol 2012;51:389-95.
[Google Scholar]
|
| 10. |
Guenova E, Volz T, Sauer K, Kaesler S, Müller MR, Wölbing F, et al. IL-4-mediated fine tuning of IL-12p70 production by human DC. Eur J Immunol 2008;38:3138-49.
[Google Scholar]
|
| 11. |
Borska L, Andrys C, Krejsek J, Hamakova K, Kremlacek J, Ettler K, et al. Serum levels of the pro-inflammatory cytokine interleukin-12 and the anti-inflammatory cytokine interleukin-10 in patients with psoriasis treated by the Goeckerman regimen. Int J Dermatol 2008;47:800-5.
[Google Scholar]
|
| 12. |
Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, Gallati H, et al. Tcells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol 1994;102:145-9.
[Google Scholar]
|
| 13. |
Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine 2015;73:342-50.
[Google Scholar]
|
| 14. |
Zhang J. Paradoxical roles of interferon-gamma in autoimmune disease. Expert Rev Clin Immunol 2007;3:35-8.
[Google Scholar]
|
| 15. |
Zhang J. Yin and yang interplay of IFN-gamma in inflammation and autoimmune disease. J Clin Invest 2007;117:871-3.
[Google Scholar]
|
| 16. |
Jones CD, Guckian M, el-Ghorr AA, Gibbs NK, Norval M. Effects of phototherapy on the production of cytokines by peripheral blood mononuclear cells and on systemic antibody responses in patients with psoriasis. Photodermatol Photoimmunol Photomed 1996;12:204-10.
[Google Scholar]
|
| 17. |
Reddy M, Torres G, McCormick T, Marano C, Cooper K, Yeilding N, et al. Positive treatment effects of ustekinumab in psoriasis: Analysis of lesional and systemic parameters. J Dermatol 2010;37:413-25.
[Google Scholar]
|
Fulltext Views
3,794
PDF downloads
1,561





