Translate this page into:
Lichen nitidus in a child receiving adalimumab for juvenile idiopathic arthritis
Corresponding author: Dr. Asunción Vicente, Department of Dermatology, Sant Joan de Déu Hospital, Universitat de Barcelona, Passeig de Sant Joan de Déu, 2, 08950 Esplugues de Llobregat, Barcelona, Spain. avicente@sjdhospitalbarcelona.org
-
Received: ,
Accepted: ,
How to cite this article: Munera-Campos M, Vicente A, Rosende L, Rovira C. Lichen nitidus in a child receiving adalimumab for juvenile idiopathic arthritis. Indian J Dermatol Venereol Leprol 2021;87:408-9.
Sir,
The use of biological agents to block specific immune system targets has been associated with onset or worsening of both organ-specific and systemic inflammatory conditions. Lichen planus and other lichenoid reactions have been reported occasionally in patients receiving antitumor necrosis factor-a agents.1 We report the first case of lichen nitidus in a child receiving adalimumab for juvenile idiopathic arthritis.
A 9-year-old boy, receiving adalimumab for juvenile idiopathic arthritis, presented with mildly pruritic skin lesions for last 1 year. Medical history was significant for oligoarticular juvenile idiopathic arthritis since 2 years age. Previous therapies included methotrexate and etanercept, none being effective. Treatment with adalimumab, started 6 months prior to skin eruption, achieved an excellent control of joint disease.
A detailed physical examination revealed multiple shiny, skin-colored, flat-topped papules measuring up to 2 mm in diameter, predominantly affecting the trunk and the extremities [Figures 1a and b]. Lesional histology demonstrated epidermal thinning and thickened papillary dermis with intense lymphocytic infiltration embraced by rete ridges, consistent with the typical ‘ball-claw’ sign [Figure 2]. Thus, we made a diagnosis of lichen nitidus. We prescribed topical methylprednisolone to obtain partial improvement.
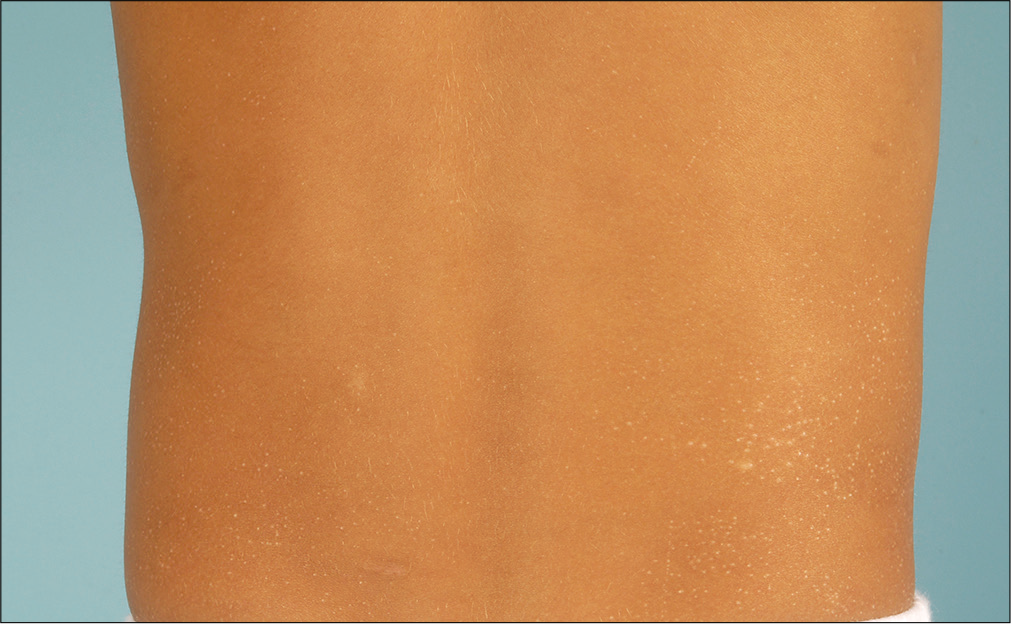
- Shiny, skin-colored, papules, affecting the trunk
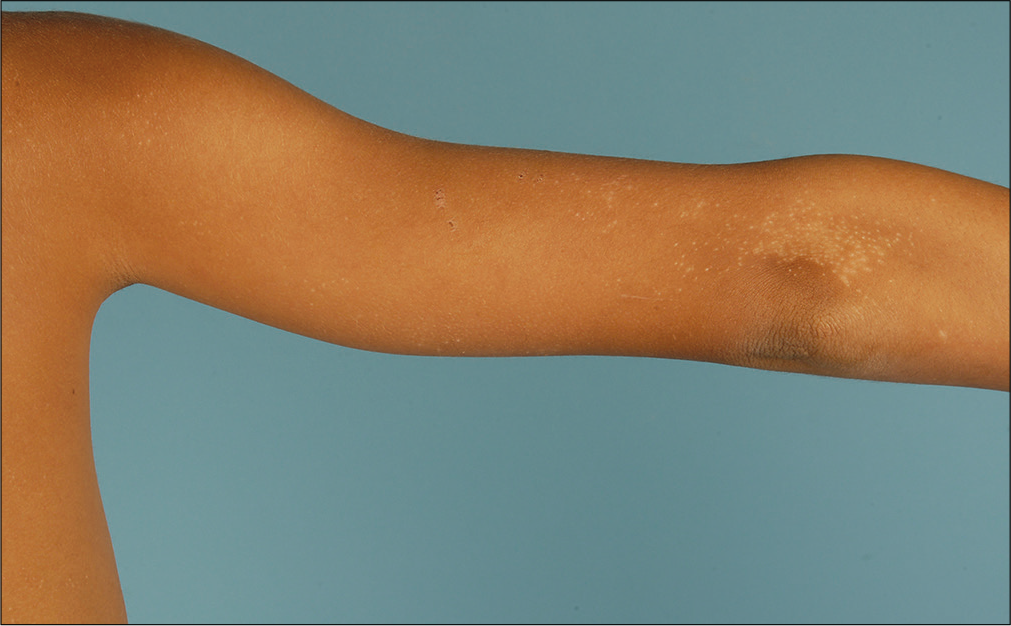
- The extensor aspect of the limb
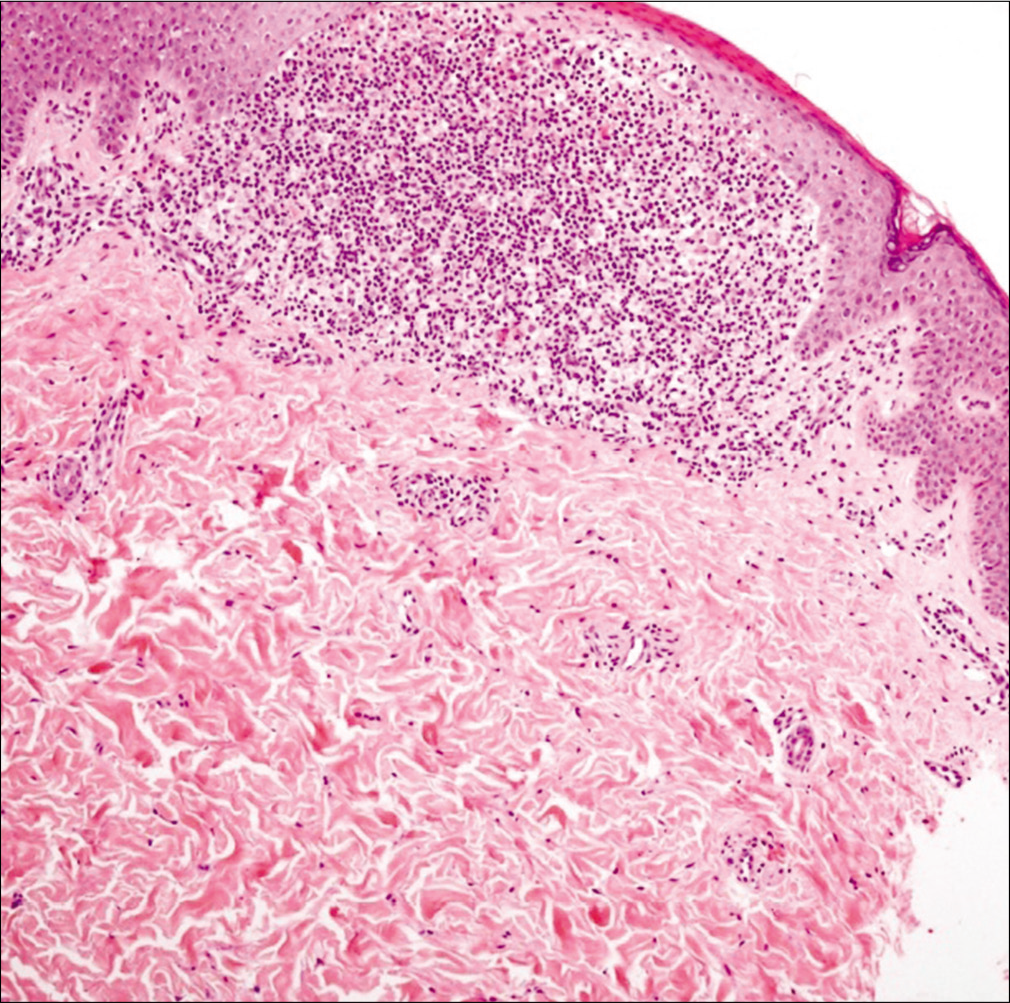
- The typical ball-claw sign in biopsy (H and E, ×400)
A year later, our patient developed an upper-tract respiratory infection, and adalimumab was discontinued, resulting in complete resolution of skin lesions by 4 weeks. After 2 months, adalimumab was restarted and a second episode of lichen nitidus developed with subsequent injections. Two years later, adalimumab was suspended due to stability of joint disease, and lichen nitidus resolved again. A joint relapse warranted re-introduction of adalimumab, leading to a third episode of lichen nitidus. Treatment with topical steroids achieved complete remission of skin lesions.
Lichen nitidus is a relatively uncommon, asymptomatic or mildly pruritic, chronic eruption, characterized by pinpoint, skin-colored or pinkish papules. It is frequently seen in children and young adults with male predilection (4:1). Its pathogenesis remains unclear, but immunological and genetic factors have been proposed. A generalized variant has been reported in children with Down syndrome, Niemann-Pick disease, and other syndromes.2
Although a single case of lichen nitidus has been reported along with juvenile idiopathic arthritis, our case was unique as all three episodes showed temporal association with an anti-tumor necrosis factor-a agent, adalimumab,3 and drug withdrawl resulted in resolution of lesions on each occasion. This finding suggests a strong causal association between adalimumab and lichen nitidus. Lichen planus and other lichenoid reactions have been reported with anti-tumor necrosis factor-a agents.4 We hypothesized that adalimumab induced cytokine imbalance to be a triggering factor for lichen nitidus. The blockade of tumor necrosis factor-a results in excess interferon-a activity, which amplifies T-cell response by induction and overexpression of chemokine receptors like CXCR3, subsequently promoting T-cell migration to other tissues, especially skin.5
We were unable to find any previous report of lichen nitidus induced by tumor necrosis factor-a blockade with adalimumab. Blockage of specific immune targets may alter the cytokine milieu, resulting in inflammatory lichenoid reactions like lichen nitidus in predisposed individuals. A detailed review of symptoms, medication and comorbidities is mandatory in such patients. Further observations are needed to confirm this association.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cutaneous side effects of anti tumor necrosis factor biologic therapy: A clinical review. J Am Acad Dermatol. 2009;61:486-504.
- [CrossRef] [PubMed] [Google Scholar]
- Generalized lichen nitidus and juvenile chronic arthritis: An undescribed association. Pediatr Dermatol. 1999;16:406-7.
- [CrossRef] [PubMed] [Google Scholar]
- Lichen planus like eruptions: An emerging side effect of tumor necrosis factor alpha antagonists. J Am Acad Dermatol. 2009;61:104-11.
- [CrossRef] [PubMed] [Google Scholar]
- Increase of peripheral CXCR3 positive T lymphocytes upon treatment of RA patients with TNF alpha inhibitors. Rheumatology (Oxford). 2005;44:172-5.
- [CrossRef] [PubMed] [Google Scholar]





