Translate this page into:
Lichen planopilaris: A review of evaluation methods
Corresponding author: Dr. Mina Saber, Department of Dermatology, Skin Diseases and Leishmaniasis Research Center, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran. mi.saber@med.mui.ac.ir
-
Received: ,
Accepted: ,
How to cite this article: Naeini FF, Saber M, Faghihi G. Lichen planopilaris: A review of evaluation methods. Indian J Dermatol Venereol Leprol 2021;87:442-5.
Introduction
Lichen planopilaris, the leading cause of scarring alopecia, was first described in 1895.1 It is characterized by a lymphocytic infiltration of hair follicles which usually affects women aged 40–60 years. Lichen planopilaris has been classified into four variants, including classic, frontal fibrosing alopecia, Graham-Little-Piccardi-Lassueur syndrome and fibrosing alopecia in a patterned distribution. The main clinical features of active disease are erythema of the scalp, perifollicular scaling, follicular hyperkeratosis and a positive anagen pull test. Most patients suffer from itching, burning and pain.2
Although the pathogenesis is not fully understood, the T-cell-mediated destruction of the hair follicles seems to suggest an underlying autoimmune etiology.2
Until now, several methods have been proposed for diagnosis or assessment of lichen planopilaris in both clinical trials and daily clinical practice, namely skin biopsy, lichen planopilaris activity index, dermoscopy, photography and reflectance confocal microscopy. Herein, we briefly review the strengths and limitations of these methods in the assessment of lichen planopilaris.
Skin Biopsy
Given the clinical similarities between lichen planopilaris and other cicatricial alopecia, such as discoid lupus erythematosus, a skin biopsy is essential for diagnosis.3 Evaluation of both vertical and horizontal sections is the preferred method for histopathological diagnosis. However, the former is more sensitive in the case of cicatricial alopecia.4
Pathologic findings vary in different stages of the disease, from perifollicular lymphocytic infiltrations, apoptosis of keratinocytes and reactive epidermal hyperplasia (early phase) to follicular plugging and loss of sebaceous glands with initial fibrosis (fully developed disease) and finally, little lymphocytic infiltration and decreased numbers of hair follicles and arrector pili muscles (late-stage).5
Skin biopsy is the most reliable and accurate method to determine the degree of inflammation and disease remission. The most important finding in active stage of the disease is a dense perifollicular infiltrate with necrotic keratinocytes. On the other hand, the disappearance of perifollicular infiltrates and the emergence of fibrosis are signs of disease remission.3,5 In other words, relieving the clinical symptoms and reducing disease activity, the two main goals for effective management are highly correlated with the degree of inflammation.2 However, being an invasive procedure, performing repeated biopsies to confirm the response to therapy is usually not feasible.
Therefore, developing standardized and practical assessment tools to capture disease activity and its changes during treatment is necessary.
Lichen Planopilaris Activity Index
Most studies that evaluated the efficacy of different medications on the clinical course of lichen planopilaris have focused on qualitative parameters such as subjective symptoms. For instance, Lyakhovitsky et al. reported clinical manifestations and response to treatment in 46 patients.1 To evaluate each patient’s response to the administered medications, they considered hair loss progression, clinical findings of active inflammation and subjective symptoms such as itching or burning. The assessment was conducted by using a qualitative scoring from 0 (no change) to 2 (improvement). Similarly, another study that evaluated the efficacy of cyclosporine in three patients with lichen planopilaris used a standardized assessment chart.6 Briefly, patients’ symptoms, hair loss status and physical examination were recorded as absent to severe. The most important pitfall of these studies was the lack of an objective end-point to clearly determine the efficacy of treatment.
To overcome this issue, a standardized index that records clinical data at each visit was introduced by Chiang et al. in 2010.7 This index which was named lichen planopilaris activity index, consisted of the patient’s symptoms (pain, itching and burning), signs (scalp erythema, perifollicular erythema and scaling), the anagen pull test and the presence or absence of spreading. Then, after assigning a score to each of these, an equation was applied to calculate the final lichen planopilaris activity index score. This was a breakthrough in designing clinical trials since by applying an overall numeric score for disease activity, a direct comparison could be made between treatments and between studies as to the effect of interventions in patients with lichen planopilaris. Including both patient symptoms and signs enabled this index to be a comprehensive tool to track the patient’s status during the treatment. In addition, its numeric system facilitated statistical analysis in comparative treatment studies.
However, the lichen planopilaris activity index has some flaws. First, using a Likert scale for rating symptoms such as pruritus or burning based on a patient’s assessment might decrease its reliability. Second, signs such as the anagen pull test which is one of the important items in the lichen planopilaris activity index, were not standardized and are variable depending on the physician’s technique. Finally, there was an interesting novelty in the original article that introduced the lichen planopilaris activity index, namely classifying the patients’ response to therapy based on the degree of lichen planopilaris activity index reduction: nonresponders (<25% reduction), partial responders (25%–85% reduction) and responders (>85% reduction).
However, some researchers believe that these cut-off points might be difficult to achieve in real daily practice, especially in the case of subacute forms of disease that constitute the most common presentation.8 Hence, they suggested modifying the above categories as failure (<25% reduction), partial responder (26%–50% reduction) and complete responder (>50% reduction). The strengths and limitations of the lichen planopilaris activity index have been summarized in Table 1.
| Strengths | Limitations |
|---|---|
| Captures several different symptoms and signs | Based on the subjective impression of patient and physician |
| Gives an overall numeric score for disease activity | Discrepancy between clinical and histological remission |
| Easily used in comparative studies | Difficult to achieve the predetermined cut-off points in clinical practice |
The lichen planopilaris activity index was used in two other studies, in 20109 and 2015.8 In the former, a retrospective study, the effect of mycophenolate mofetil was assessed in 16 patients. The latter research was a clinical trial in 60 patients that compared the efficacy of topical clobetasol with oral mycophenolate mofetil on disease activity. Recently, we conducted a randomized clinical trial to compare the efficacy of hydroxychloroquine with methotrexate, and we used the lichen planopilaris activity index to monitor the patients.10 To track disease activity during the study, pre- and post-treatment changes in mean lichen planopilaris activity index were compared instead of the categorization introduced by Chiang et al. (mentioned above). If we had applied the cut-off points of lichen planopilaris activity index reduction to classify the response to treatment (unpublished data), the better therapeutic profile of methotrexate over hydroxychloroquine would have been overlooked. This observation may be explained by the strong effect of the anagen pull test and spreading on the lichen planopilaris activity index calculation. Their approximate weight in total lichen planopilaris activity index is 40%.
In other words, in cases of patients with subacute forms that do not usually show these two signs, their lichen planopilaris activity index might be low at baseline and achievement of a >85% reduction in lichen planopilaris activity index would be an unachievable goal. Therefore, we suggest that a comparison of mean lichen planopilaris activity index changes may be more reasonable than using the above mentioned cut-off points in clinical trials.
Dermoscopy
Scalp dermoscopy (trichoscopy) has been used recently for diagnosis and treatment monitoring of alopecias.11 Several recent studies have addressed dermoscopic findings in lichen planopilaris. In active disease, perifollicular scaling and follicular keratotic plugs are observed, and in the late and fibrotic stage, irregular and large white dots with atrophic smooth pale skin have been reported.11,12
Correct selection of biopsy sites is another important issue in the diagnosis of cicatricial alopecia such as lichen planopilaris. Miteva et al. showed that dermoscopy-guided biopsy from areas of perifollicular concentric white scales might demonstrate a higher yield of diagnosis.13 Moreover, the application of interventions such as mesotherapy or intralesional corticosteroids could be more directed if active areas of inflammation could be identified by dermoscopy. In addition, dermoscopy can reveal minor changes that are not visible to the naked eye such as those in subepidermal structures.14 For instance, perifollicular scaling which corresponds to vacuolar degeneration in the basal layer of the outer hair follicle root sheath, might easily be overlooked in very early and subacute forms of the disease.15
Finally, it could be helpful to differentiate lichen planopilaris from other cicatricial alopecia such as discoid lupus erythematosus.11
Photography
Photography is another noninvasive assessment tool for evaluating the response to therapy in patients with lichen planopilaris. One of the most popular methods of photography is the Canfield method.16 However, since it relies on subjective symptoms of inflammation, it is not suitable for accurate disease monitoring. Hence, it will be more informative if we instead consider the “hair counting technique.”
In this method, changes in the hair counts in a marked area are measured pre- and post-treatment [Figure 1].17 Since it is based on objective signs (hair count), this method may be reliable and practical in the setting of clinical trials.

- Photographic assessment. The most active area was marked. Pretreatment: severe perifollicular scaling and pigmentation with skin erythema were seen
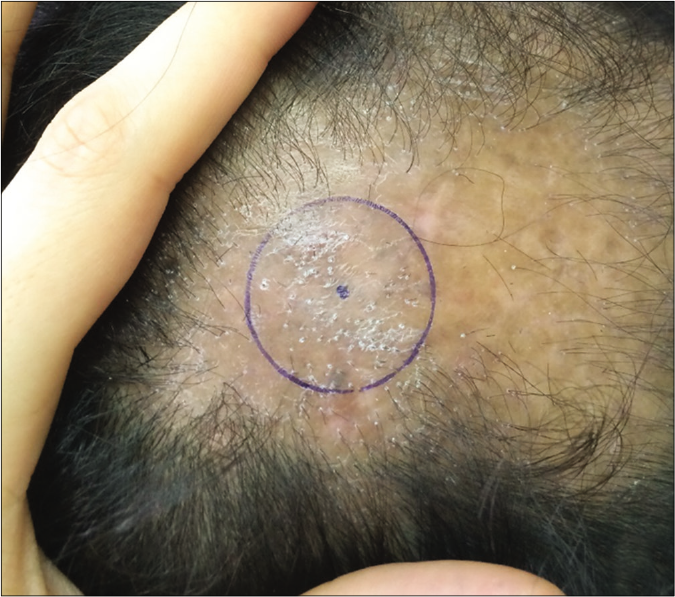
- Photographic assessment. The most active area was marked. Posttreatment: decreased in inflammatory signs especially perifollicular scaling were observed
Reflectance Confocal Microscopy
Since the above mentioned methods cannot be used to evaluate the microscopic appearance of skin lesions, reflectance confocal microscopy which has been used previously for evaluation of inflammatory and malignant skin lesions, was introduced for hair and scalp disorders such as lichen planopilaris. It can be used to demonstrate the degree of inflammatory infiltrates in scalp disease and for monitoring the response to therapy.18 However, there is still a need for more studies to delineate the real role and efficacy of reflectance confocal microscopy to monitor patients with lichen planopilaris.
In summary, we addressed the strengths and limitations of each assessment method for lichen planopilaris. Although introduction of lichen planopilaris activity index is an advance in the evaluation of these patients, it cannot always provide an accurate estimation of disease activity. Therefore, a combination of the aforementioned tools in clinical practice and research studies may improve our capabilities in the assessment of treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- A case series of 46 patients with lichen planopilaris: Demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-9.
- [CrossRef] [PubMed] [Google Scholar]
- Lichen planopilaris: Update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
- [CrossRef] [PubMed] [Google Scholar]
- A histologic review of 27 patients with lichen planopilaris. J Am Acad Dermatol. 2008;59:91-8.
- [CrossRef] [PubMed] [Google Scholar]
- Vertical vs. transverse sections of scalp biopsy specimens: A pilot study on the comparison of the diagnostic value of two techniques in alopecia. Clin Exp Dermatol. 2011;36:855-63.
- [CrossRef] [PubMed] [Google Scholar]
- Lichen planopilaris-histologic criteria & clues in vertical sections. Hair Ther Transplant. 2013;3:2167-0951.10001.
- [Google Scholar]
- Short course of oral cyclosporine in lichen planopilaris. J Am Acad Dermatol. 2003;49:667-71.
- [CrossRef] [Google Scholar]
- Hydroxychloroquine and lichen planopilaris: Efficacy and introduction of lichen planopilaris activity index scoring system. J Am Acad Dermatol. 2010;62:387-92.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of systemic mycophenolate mofetil with topical clobetasol in lichen planopilaris: A parallel-group, assessor-and analyst-blinded, randomized controlled trial. Am J Clin Dermatol. 2015;16:303-11.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of mycophenolate mofetil for lichen planopilaris. J Am Acad Dermatol. 2010;62:393-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical efficacy and safety of methotrexate versus hydroxychloroquine in preventing lichen planopilaris progress: A randomized clinical trial. Int J Prev Med. 2017;8:37.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy patterns of cicatricial alopecia resulting from discoid lupus erythematosus and lichen planopilaris. An Bras Dermatol. 2010;85:179-83.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopic findings in different clinical variants of lichen planus. Is dermoscopy useful? Dermatol Pract Concept. 2015;5:51-5.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy guided scalp biopsy in cicatricial alopecia. J Eur Acad Dermatol Venereol. 2013;27:1299-303.
- [CrossRef] [PubMed] [Google Scholar]
- Invisible lichen planopilaris unmasked by dermatoscopy. Int J Trichology. 2017;9:76-8.
- [Google Scholar]
- Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-21.
- [CrossRef] [Google Scholar]
- Clinical and photographic assessment of lichen planopilaris treatment efficacy. J Am Acad Dermatol. 2011;64:597-8.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic follow-up of lichen planopilaris using in vivo reflectance confocal microscopy: A case report. Skin Res Technol. 2015;21:380-3.
- [CrossRef] [PubMed] [Google Scholar]





