Translate this page into:
Linking of psoriasis with osteopenia and osteoporosis: A cross-sectional study
2 General Medicine Department, Community Medicine Unit, Virgen de las Nieves University Hospital, Granada, Spain
3 Osteoporosis Department, Endocrinology Unit, Virgen de las Nieves University Hospital, Granada, Spain
4 General Orthophedics Department, Orthopedics Unit, Virgen de las Nieves University Hospital, Granada, Spain
5 Dermatology Department, Medicine School of the University of Granada, Granada, Spain
6 General Dermatology Department, Dermatology Unit, Virgin de las Nieves University Hospital; Dermatology Department, Medicine School of the University of Granada, Granada, Spain
Correspondence Address:
Antonio Martinez-Lopez
Av de la Ilustracion, 65, 5°A, 18016, Granada
Spain
| How to cite this article: Martinez-Lopez A, Blasco-Morente G, Giron-Prieto MS, Arrabal-Polo MA, Luque-Valenzuela M, Luna-Del Castillo JD, Tercedor-Sanchez J, Arias-Santiago S. Linking of psoriasis with osteopenia and osteoporosis: A cross-sectional study. Indian J Dermatol Venereol Leprol 2019;85:153-159 |
Abstract
Background/Purpose: Psoriasis is a multisystem disease which has been related to vitamin-D deficiency through chronic inflammation. This psoriasis-related inflammatory state and vitamin-D deficiency may induce bone mineral density loss. The purpose of this study is to assess the relationship of psoriasis with bone mineral density, by comparing psoriatic patients with healthy controls and patients with osteopenia/osteoporosis.
Methods: A total of 185 subjects were studied; 58 psoriatic patients who had not been under systemic or biological treatment were included. Age, gender, body mass index, phosphocalcic metabolic parameters and hip and lumbar (L4) bone mineral density data were collected. These variables were compared with those collected in 61 healthy controls and 67 patients with osteopenia/osteoporosis.
Results: Psoriatic patients showed worse hip and lumbar spine bone mineral density levels than healthy controls (P = 0.001) and better levels than osteoporotic patients (P < 0.001). Multivariate analysis demonstrated a negative association of age and a positive association of body mass index in hip bone mineral density in psoriatic patients.
Limitations: The main limitations are those of cross-sectional studies, such as a lack of follow up period, and a male predominance in the psoriatic group, which is corrected employing a multivariate analysis with an adjusted model for confounding factors.
Conclusions: Bone mineral density levels in psoriatic patients are situated halfway between healthy controls and patients with osteopenia/osteoporosis. In addition, the higher body mass index in patients with psoriasis appears to confer a protective effect against further development of lower bone mineral density.
Introduction
Psoriasis is a chronic inflammatory disease whose prevalence is estimated to be between 1 and 5% of the world population.[1] This condition has been associated with a higher prevalence of metabolic syndrome, obesity, hyperlipidemia, diabetes mellitus, hypertension and arteriosclerosis.[2],[3] These comorbidities seem to be responsible for the increased risk of major cardiovascular events in psoriatic patients.[4] Among the comorbidities associated with psoriasis, the association of this disease with vitamin D deficiency is globally recognized. The presence of lower levels of vitamin D in psoriatic patients have been related with higher levels of cholesterol, low-density lipoprotein, triglycerides and glucose.[5],[6] The reason for such a large number of pathologies associated with psoriasis appears to lie in the chronic inflammatory state present in psoriatic patients, which has been demonstrated by increased proinflammatory cytokines such as tumor necrosis factor-alpha, interleukin-12, interleukin-23 or interleukin-17 in these patients.[7],[8]
Moreover, increased levels of C-reactive protein have been found in psoriatic patients, as well as low levels of vitamin-D metabolizing enzymes, CYP27A1 and CYP27B1, within psoriatic lesions.[9],[10] On the other hand, vitamin-D supplementation and treatment with oral calcitriol have been associated with clinical improvement in psoriasis lesions.[11],[12],[13]
Osteoporosis is a skeletal disorder characterized by reduced bone mass and micro - architectural changes in bone tissue that result in an increased risk of fractures.[14] The World Health Organization defines osteoporosis as bone mineral density <2.5 standard deviation below the mean for young white adult women (T-score). Osteopenia is defined as the presence of a bone mineral density between 1.5 and 2.5 below the T-score and represents a low bone mass that is not as severe as osteoporosis. This entity is a major public health problem due to the increased risk of development of fractures associated with osteoporosis.[15] However, there is another type of osteoporosis that can occur secondary to various entities such as endocrine pathology, rheumatologic disease, increased chronic inflammation and vitamin-D deficiency.[16] In addition, the presence of a lower bone mineral density has been described in patients with long-term psoriasis in a study without a control group.[17]
Considering this background, a cross-sectional study with a control group was conducted to assess the levels of bone mineral density in patients with psoriasis in comparision to those existing in healthy controls and patients without psoriasis diagnosed with osteopenia or osteoporosis.
Methods
Patients with moderate to severe cutaneous psoriasis were systematically recruited from the outpatient department of the Psoriasis Unit of our hospital. Inclusion criteria were: a clinical diagnosis of psoriasis with a Psoriasis Area Severity Index higher than 4, age 18 years or older, residence in the metropolitan area of Granada (southern Spain) and adequate daily sun exposure, described as staying outdoors for at least 1 hour per day. Exclusion criteria were: personal history of treatment with systemic or biologic agents, or photochemotherapy, personal history of psoriatic arthritis, rheumatoid arthritis, inflammatory bowel disease or other inflammatory diseases and intake of calcium or vitamin-D supplements. Moreover, healthy controls from the same metropolitan area and patients diagnosed with osteopenia or osteoporosis who were followed in the Endocrinology Unit of our hospital were included in the study. All the osteopenia/osteoporosis patients had been recently diagnosed by densitometry and had not received any kind of treatment. Patients who met these selection criteria and signed an informed consent (in accordance with the Helsinki Declaration) were enrolled in the study; no selected patients refused to participate. The study was approved by the Ethics Committee of Hospital Universitario Virgen de las Nieves, Granada, Spain.
Clinical and laboratory parameters
Data regarding age and sex were collected from all study participants. All patients underwent physical examination, and their weight and height were recorded to calculate their body mass index (kg/m2), as well as their psoriasis area and severity index. Blood samples were drawn in the early morning for laboratory analysis of biochemical parameters, including serum 25(OH) vitamin-D, calcium, phosphorus, parathyroid hormone and osteocalcin. In addition, bone mineral density was measured by bone densitometry using dual-energy X-ray absorptiometry, employing an Hologic Discovery Wi® (Hologic Inc., USA).T-score values in the Ward triangle of the Hip (tHip), the fourth vertebra in the lumbar spine (tl4) and the whole lumbar spine (tl1-4) were obtained. All laboratory parameters were collected in two consecutive months (March/April 2016).
Statistical analysis
A descriptive analysis by group, psoriasis (P), control (C) and osteopenia/osteoporosis (O), was carried out for all the variables in database. Contingency tables according to group and sex were made using generalization of exact Fisher's test in RXC tables to assess the association between them. One-way analysis of variance was performed for each numerical variable to compare means by group and stratified by sex. Correlation matrix between numerical variables was computed to measure the association between them.
To measure mean differences between P–C and P–O, multiple linear regression was computed. Given that differences between groups were affected by confounding factors (sex, body mass index, vitamin-D, parathyroid hormone and osteocalcin), a crude and an adjusted model are presented, the latter being the main analysis of the study. This type of analysis allows the elimination of existing differences between the various study groups. The outcomes considered were tHip and tl4, and covariates were group, sex, body mass index, vitamin D, parathyroid hormone and osteocalcin.
Fore statistical analysis, STATA 14.1 (Statacorp, College Station, Texas, EEUU) was used.
Results
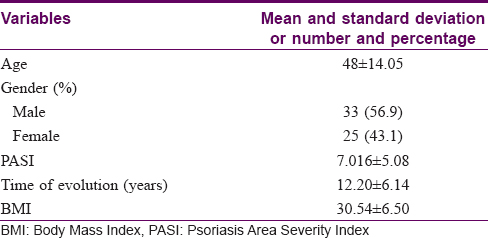
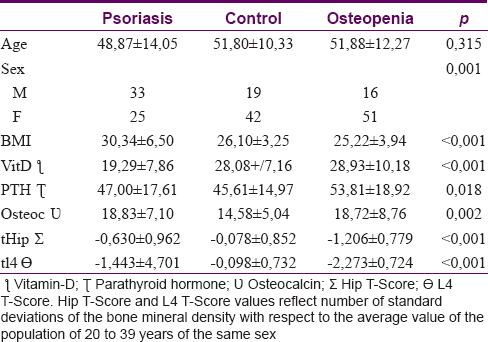
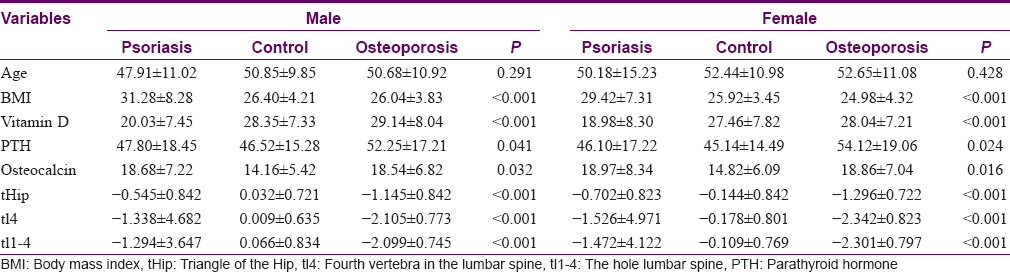
The final study sample of 185 patients comprised three groups – the psoriasis group (P group) with 58 patients [33 males and 25 females, [Table - 1], the control group (C group) with 61 patients (19 male and 42 female) and the osteopenia/osteoporosis group (O group) with 67 patients (16 male and 51 female). The three groups [Table - 2] differed in the number of males and females included in each group (P < 0.001); however, no differences were found in mean age (P group: 48.88 ± 14.04; C group: 51.80 ± 10.33; O group: 51.88 ± 12.27; P = 0.315). Body mass index was found to be higher in P group than the other groups (P: 30.34 ± 6.50; C: 26.10 ± 3.25; O: 25.22 ± 3.94; P < 0.001). Moreover, vitamin-D levels were lower in P group in relation to the other groups (P < 0.001). In addition, differences in the parathyroid hormone means were found, showing upper levels in the O group with respect to the other two groups (P = 0.018) and higher osteocalcin levels in the P and O groups compared to the C group (P = 0.002). Finally, the crude comparison of means concerning the outcomes tHip and tl4 showed significative differences (P < 0.001) between different groups. These statistical differences remained stable after stratification by sex [Table - 3].
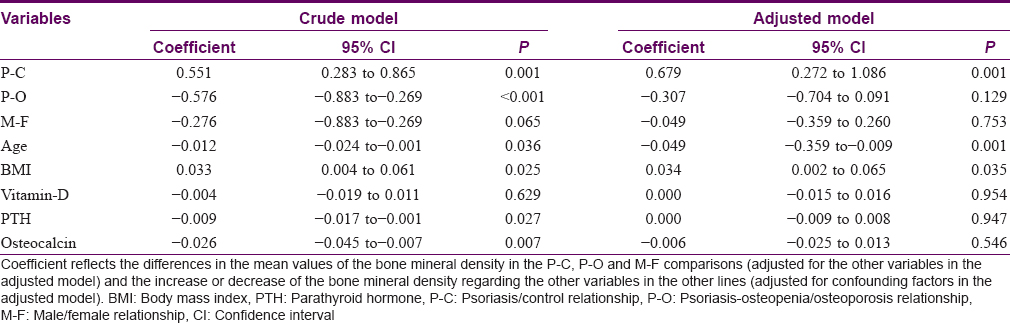
The multiple linear regression for hip T-score adjusted for confounding factors, including sex, age, body mass index, vitamin-D, parathyroid hormone and osteocalcin [Table - 4], showed that the mean hip T-score in group C was higher than group P (P = 0.001), whereas the average T-score in the O group was lower than P group, not reaching statistical significance (P = 0.129). Furthermore, age showed an independent effect on the levels of hip T-score, decreasing its value with the increased age of patients (P = 0.001). Furthermore, the body mass index variable also showed a significant effect on hip T-score, increasing its value with the body mass index rising [r = 0.272; P = 0.035, [Figure - 1]. On the contrary, an inverse association between hip T-score and Psoriasis Area Severity Indexwas found, although this association was nearly significant [r= −0.230; P = 0.091, [Figure - 2]. The variables such as gender, vitamin-D, parathyroid hormone and osteocalcin did not show any significant effect on hip T-score.
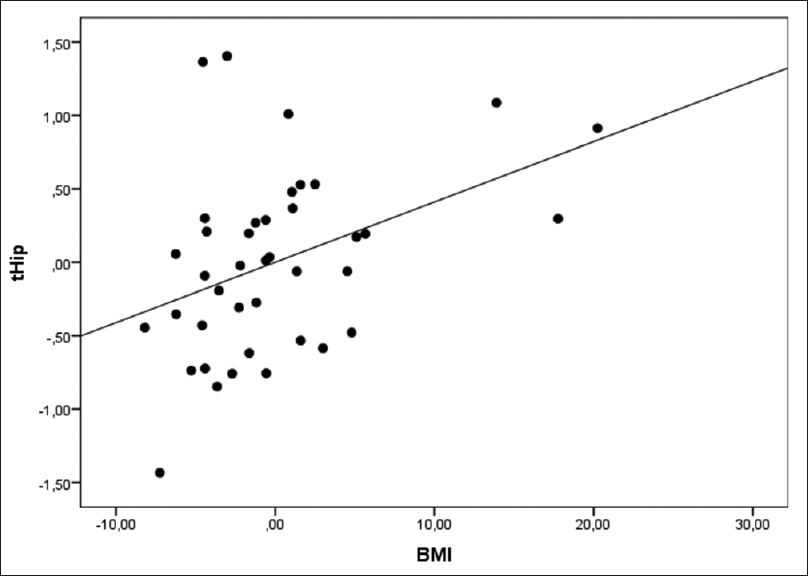 |
| Figure 1: Correlation between body mass index and hip T-score (r = 0.272) |
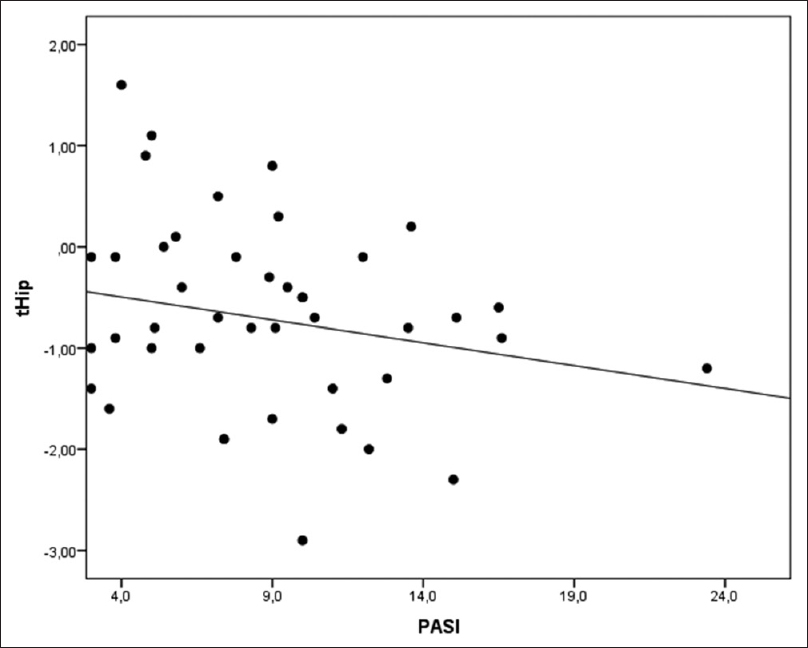 |
| Figure 2: Correlation between psoriasis area severity index and hip T-score (r = −0.230) |
The multiple linear regression for L4 T-score adjusted for confounding factors [Table - 5] revealed that the average T-score in C was higher thanP (P = 0.002), while the mean T-score in O was significantly lower than P (P < 0.001). None of the other variables showed a significant effect on L4 T-score.

Discussion
General data
In this study, bone mineral density values in patients with psoriasis were significantly lower than in healthy controls from the general population. In addition, a trend was observed in psoriasis patients, who presented with better levels of bone mineral density than patients of osteopenia/osteoporosis. Significant differences between group P and group O were only observed in the T-score of L4 after adjustment for confounding factors.
Although not related to the primary objective of the study, some of the current results deserve comment. First, in the psoriatic group the percentage of men was slightly higher than women (56.9% vs 43.1%), while in the control group and osteoporosis group the percentage of women was higher (68.9 and 76.1%, respectively). These results are consistent with the findings of a recent American review[18] and another study carried out in Spanish population.[19] On the contrary, the high percentage of women in the O group is similar to that found in other epidemiological studies in industrialized countries.[20],[21] Likewise, a higher body mass index in psoriatic patients has also been described.[22] In addition, previous studies have demonstrated that increased body mass index was positively correlated with increased risk of developing psoriasis.[23],[24]
Relationship between psoriasis and lower levels of bone mineral density
In our study, we have observed lower levels of bone mineral density in patients with psoriasis as compared to healthy controls. There are several studies that reinforce the idea of the association of psoriasis with lower levels of bone mineral density. A recent cross-sectional study without control group performed in patients with psoriasis and psoriatic arthritis showed that 63% of patients had deficient levels of vitamin-D, which is inversely correlated with body mass index of patients.[25] In addition, in this study no significant association between vitamin-D levels and bone mineral density was found, which is consistent with our findings. In 2011, Attia et al. demonstrated that patients with psoriasis and/or psoriatic arthritis showed worse bone mineral density levels relative to healthy controls.[26] Similar results have been recently published in a study performed in Turkish population.[27] In this paper, the presence of lower levels of bone mineral density in women with psoriasis relative to healthy controls is described, while no differences were found in the comparison between men. However, both these studies did not perform an adjustment for possible confounders such as body mass index or age.
An inverse association between body mass index and serum levels of vitamin D have been demonstrated by several studies, both in patients with psoriasis[7],[25] and in healthy and obese patients.[28],[29],[30]
Correlation between body mass index and bone mineral density
This possible association between the two parameters could suggest that patients with psoriasis and high body mass index have lower levels of bone mineral density at the expense of reduced serum levels of vitamin-D. However, in our study we have found a positive association between body mass index and hip T-score in patients with psoriasis. These results are consistent with other publications, in which it is described that a low body mass index correlates with lower bone mineral density in postmenopausal women.[31] In 2011, Pedreira et al. conducted a cross-sectional study with a control group, comparing the association of bone mineral density levels with various anthropometric parameters in patients with psoriasis.[32] In this paper, a positive correlation between bone mineral density and body mass index was observed. Recently, Honma et al. have reported a similar positive correlation in psoriatic patients.[33] In addition, Skrzek et al. have determined that the risk of developing osteopenia/osteoporosis in postmenopausal women is lower in those with a body mass index of 26–27.9.[34] Thus, after adjustment for confounding factors, the high body mass index present in patients with psoriasis appears to protect these patients against osteopenia/osteoporosis, mitigating the negative effect on bone mineral density produced by chronic inflammation and low levels of vitamin D present therein. This protective effect is particularly evident by observing the lower difference in bone mineral density between the psoriatic group and the osteopenia/osteoporosis group after the adjustment.
The association between psoriasis and chronic inflammation has been extensively described in literature, being one of the most common immune-mediated inflammatory skin disorders.[35] This increase in systemic chronic inflammation has also been related with lower vitamin-D and bone mineral density levels.[7],[17]
Limitations and need for future studies
The limitations of this study are those of cross-sectional studies with control group, such as the lack of follow up and the inability of making causality inferences. Although there is a male predominance in the P group, the use of a multivariate analysis with adjusted model eliminates the differences between the study groups mediated by this variable, making the groups fully comparable. On the contrary, despite the multiple studies describing the association between psoriasis and low bone mineral density levels, a recent Norwegian population-based study has not found a reduced bone mineral density level or an increased risk of osteoporosis in psoriatic patients.[36] Moreover, despite the deficit of vitamin D found in patients with psoriasis, treatment with oral calcium and/or vitamin D to prevent osteoporotic fractures remains controversial.[37],[38]
Conclusions
In conclusion, a cross-sectional study comparing bone mineral density in psoriatic patients vshealthy controls and patients with osteopenia/osteoporosis is presented. We were unable to find any report of the last comparison in literature. According to our results, levels of bone mineral density in patients with moderate to severe psoriasis are situated halfway between those present in healthy controls and patients with osteopenia/osteoporosis. The higher body mass index found in the psoriatic group could be a protective factor against the development of osteopenia/osteoporosis in these patients. However, more studies would be needed to demonstrate a causal association between psoriasis and the risk of developing osteopenia/osteoporosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Acknowledgement
To Alberto Andrés, for its help with English translation.
Financial support and sponsorship
The authors declare not to have any financial support and sponsorship
Conflicts of interest
There are no conflicts of interest.
| 1. |
Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370:263-71.
[Google Scholar]
|
| 2. |
Arias-Santiago S, Orgaz-Molina J, Castellote-Caballero L, Arrabal-Polo MÁ, García-Rodriguez S, Perandrés-López R, et al. Atheroma plaque, metabolic syndrome and inflammation in patients with psoriasis. Eur J Dermatol 2012;22:337-44.
[Google Scholar]
|
| 3. |
Menter A, Griffiths CE, Tebbey PW, Horn EJ, Sterry W; International Psoriasis Council, et al. Exploring the association between cardiovascular and other disease-related risk factors in the psoriasis population: The need for increased understanding across the medical community. J Eur Acad Dermatol Venereol 2010;24:1371-7.
[Google Scholar]
|
| 4. |
Egeberg A, Bruun LE, Mallbris L, Gislason GH, Skov L, Wu JJ, et al. Family history predicts major adverse cardiovascular events (MACE) in young adults with psoriasis. J Am Acad Dermatol 2016;75:340-6.
[Google Scholar]
|
| 5. |
Orgaz-Molina J, Magro-Checa C, Rosales-Alexander JL, Arrabal-Polo MA, Buendía-Eisman A, Raya-Alvarez E, et al. Association of 25-hydroxyvitamin D serum levels and metabolic parameters in psoriatic patients with and without arthritis. J Am Acad Dermatol 2013;69:938-46.
[Google Scholar]
|
| 6. |
Hmamouchi I, Paternotte S, Molto A, Etcheto A, Borderie D, Combe B, et al. Vitamin D, disease activity and comorbidities in early spondyloarthritis. Clin Exp Rheumatol 2016;34:396-403.
[Google Scholar]
|
| 7. |
Kimura Y, Shimada-Omori R, Takahashi T, Tsuchiyama K, Kusakari Y, Yamasaki K, et al. Therapeutic drug monitoring of patients with psoriasis during tumour necrosis factor (TNF)-α antagonist treatment using a novel interleukin-8 reporter cell line. Br J Dermatol 2016;175:979-87.
[Google Scholar]
|
| 8. |
Senra L, Stalder R, Alvarez Martinez D, Chizzolini C, Boehncke WH, Brembilla NC, et al. Keratinocyte-derived IL-17E contributes to inflammation in psoriasis. J Invest Dermatol 2016;136:1970-80.
[Google Scholar]
|
| 9. |
Orgaz-Molina J, Magro-Checa C, Arrabal-Polo MA, Raya-Álvarez E, Naranjo R, Buendía-Eisman A, et al. Association of 25-hydroxyvitamin D with metabolic syndrome in patients with psoriasis: A case-control study. Acta Derm Venereol 2014;94:142-5.
[Google Scholar]
|
| 10. |
Ala-Houhala MJ, Karppinen T, Vähävihu K, Kautiainen H, Dombrowski Y, Snellman E, et al. Narrow-band ultraviolet B treatment boosts serum 25-hydroxyvitamin D in patients with psoriasis on oral Vitamin D supplementation. Acta Derm Venereol 2014;94:146-51.
[Google Scholar]
|
| 11. |
Gaál J, Lakos G, Szodoray P, Kiss J, Horváth I, Horkay E, et al. Immunological and clinical effects of alphacalcidol in patients with psoriatic arthropathy: Results of an open, follow-up pilot study. Acta Derm Venereol 2009;89:140-4.
[Google Scholar]
|
| 12. |
Trémezaygues L, Reichrath J. Vitamin D analogs in the treatment of psoriasis: Where are we standing and where will we be going? Dermatoendocrinol 2011;3:180-6.
[Google Scholar]
|
| 13. |
Millsop JW, Bhatia BK, Debbaneh M, Koo J, Liao W. Diet and psoriasis, part III: Role of nutritional supplements. J Am Acad Dermatol 2014;71:561-9.
[Google Scholar]
|
| 14. |
NIH consensus development panel on osteoporosis prevention, diagnosis, and therapy, March 7-29, 2000: Highlights of the conference. South Med J 2001;94:569-73.
[Google Scholar]
|
| 15. |
Drake MT, Clarke BL, Lewiecki EM. The pathophysiology and treatment of osteoporosis. Clin Ther 2015;37:1837-50.
[Google Scholar]
|
| 16. |
Emkey GR, Epstein S. Secondary osteoporosis: Pathophysiology and diagnosis. Best Pract Res Clin Endocrinol Metab 2014;28:911-35.
[Google Scholar]
|
| 17. |
D'Epiro S, Marocco C, Salvi M, Mattozzi C, Luci C, Macaluso L, et al. Psoriasis and bone mineral density: Implications for long-term patients. J Dermatol 2014;41:783-7.
[Google Scholar]
|
| 18. |
Andersen LK, Davis MD. Sex differences in the incidence of skin and skin-related diseases in Olmsted County, Minnesota, United States, and a comparison with other rates published worldwide. Int J Dermatol 2016;55:939-55.
[Google Scholar]
|
| 19. |
Ferrándiz C, Carrascosa JM, Toro M. Prevalence of psoriasis in spain in the age of biologics. Actas Dermatosifiliogr 2014;105:504-9.
[Google Scholar]
|
| 20. |
Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O'Malley CD. Estimating prevalence of osteoporosis: Examples from industrialized countries. Arch Osteoporos 2014;9:182.
[Google Scholar]
|
| 21. |
Fujiwara S. Epidemiology of osteoporosis in men. Clin Calcium 2016;26:1003-8.
[Google Scholar]
|
| 22. |
Lønnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF, et al. Association of psoriasis with the risk for type 2 diabetes mellitus and obesity. JAMA Dermatol 2016;152:761-7.
[Google Scholar]
|
| 23. |
Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A, et al. Prevalence of metabolic syndrome in patients with psoriasis: A hospital-based case-control study. Br J Dermatol 2007;157:68-73.
[Google Scholar]
|
| 24. |
Brauchli YB, Jick SS, Meier CR. Psoriasis and the risk of incident diabetes mellitus: A population-based study. Br J Dermatol 2008;159:1331-7.
[Google Scholar]
|
| 25. |
Kincse G, Bhattoa PH, Herédi E, Varga J, Szegedi A, Kéri J, et al. Vitamin D3 levels and bone mineral density in patients with psoriasis and/or psoriatic arthritis. J Dermatol 2015;42:679-84.
[Google Scholar]
|
| 26. |
Attia EA, Khafagy A, Abdel-Raheem S, Fathi S, Saad AA. Assessment of osteoporosis in psoriasis with and without arthritis: Correlation with disease severity. Int J Dermatol 2011;50:30-5.
[Google Scholar]
|
| 27. |
Solak B, Dikicier BS, Celik HD, Erdem T. Bone mineral density, 25-OH Vitamin D and inflammation in patients with psoriasis. Photodermatol Photoimmunol Photomed 2016;32:153-60.
[Google Scholar]
|
| 28. |
Osmancevic A, Gillstedt M, Landin-Wilhelmsen K, WennbergLarkö AM, Larkö O, Holick MF, et al. Size of the exposed body surface area, skin erythema and body mass index predict skin production of Vitamin D. J Photochem Photobiol B 2015;149:224-9.
[Google Scholar]
|
| 29. |
Shantavasinkul PC, Phanachet P, Puchaiwattananon O, Chailurkit LO, Lepananon T, Chanprasertyotin S, et al. Vitamin D status is a determinant of skeletal muscle mass in obesity according to body fat percentage. Nutrition 2015;31:801-6.
[Google Scholar]
|
| 30. |
Samuel L, Borrell LN. The effect of body mass index on adequacy of serum 25-hydroxyvitamin D levels in US adults: The national health and nutrition examination survey 2001 to 2006. Ann Epidemiol 2014;24:781-4.
[Google Scholar]
|
| 31. |
Wu SF, Du XJ. Body mass index may positively correlate with bone mineral density of lumbar vertebra and femoral neck in postmenopausal females. Med Sci Monit 2016;22:145-51.
[Google Scholar]
|
| 32. |
Pedreira PG, Pinheiro MM, Szejnfeld VL. Bone mineral density and body composition in postmenopausal women with psoriasis and psoriatic arthritis. Arthritis Res Ther 2011;13:R16.
[Google Scholar]
|
| 33. |
Honma M, Shibuya T, Iinuma S, Kishibe M, Takahashi H, Ishida-Yamamoto A, et al. Close correlation of bone mineral density and body mass index in Japanese psoriasis patients. J Dermatol 2017;44:e1-2.
[Google Scholar]
|
| 34. |
Skrzek A, Kozieł S, Ignasiak Z. The optimal value of BMI for the lowest risk of osteoporosis in postmenopausal women aged 40-88 years. Homo 2014;65:232-9.
[Google Scholar]
|
| 35. |
Ueyama A, Imura C, Fusamae Y, Tsujii K, Furue Y, Aoki M, et al. Potential role of IL-17-producing CD4/CD8 double negative αβ T cells in psoriatic skin inflammation in a TPA-induced STAT3C transgenic mouse model. J Dermatol Sci 2017;85:27-35.
[Google Scholar]
|
| 36. |
Modalsli EH, Šsvold BO, Romundstad PR, Langhammer A, Hoff M, Forsmo S, et al. Psoriasis, fracture risk and bone mineral density: The HUNT study, norway. Br J Dermatol 2017;176:1162-9.
[Google Scholar]
|
| 37. |
Bauer DC. Calcium supplements and fracture prevention. N Engl J Med 2014;370:387-8.
[Google Scholar]
|
| 38. |
Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med 2016;374:254-62.
[Google Scholar]
|
Fulltext Views
5,781
PDF downloads
2,116





