Translate this page into:
Mammalian target of rapamycin (mTOR) inhibitors in dermatology
Corresponding author: Dr. Anand M, Department of Dermatology, Armed Forces Medical College, Pune, Maharashtra, India. anandstanley09@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mannu A, Neema S, Vasudevan B, Bhatt S. Mammalian target of rapamycin (mTOR) inhibitors in dermatology. Indian J Dermatol Venereol Leprol 2023;89:767-70
Introduction
The mammalian target of rapamycin (mTOR) is a serine-threonine kinase, which has been extensively studied in the last three decades and has paved the way for a better understanding of pathogenesis and exploration of new treatment modalities of many skin diseases. In 1965, Sehgal et al. isolated rapamycin (sirolimus), a fermented product of Streptomyces hygroscopicus, from soil samples of Eastern Island (Rapa Nui) collected by Canadian explorers. 1 Initially, it was thought to have antifungal properties, but later its immunosuppressive, antiproliferative and antiangiogenic properties came to light. The mammalian target of rapamycin inhibitors (mTORi) are exciting molecules being explored for various uses in dermatology. 2
Mammalian target of rapamycin pathway
The mTOR protein belongs to the phosphoinositide 3-kinase (PI3K) family and its signalling pathway act as a key regulator in cell metabolism, proliferation and differentiation, immune system modulation and autophagy mechanisms. 3 The mTOR protein binds with other proteins to form two multiprotein complexes, the mTOR-complex 1 and the mTOR-complex 2, with different compositions and signalling functions. As explained in Figure 1, mTOR-complex 1 is rapamycin-sensitive and sensitive to hypoxia, and nutrient deficiency deoxyribonucleic acid damage. It consists of positive regulators like G-protein β-subunit-like protein and two negative regulators proline-rich Akt substrate 40 kDa, and DEP domain-containing mTOR-interacting protein. The mTOR-complex 2 is a rapamycin-insensitive complex and consists of a rapamycin-insensitive companion of the m-TOR, mammalian stress-activated protein kinase interacting protein), G protein β subunit-like protein and DEPTOR.
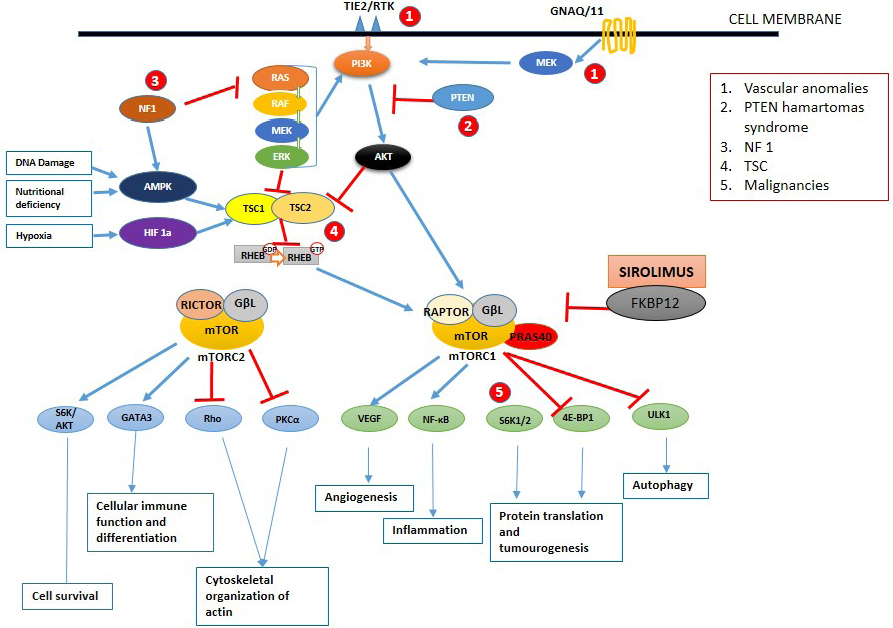
- Schematic diagram of the mTOR signalling pathway. Once activated by phosphoinositide 3-kinase, the mammalian target of rapamycin works by activating two different complexes resulting in various functions. Blue and red arrows indicate stimulation and inhibition, respectively. AKT: protein kinase B, AMPK-5: AMP-activated protein kinase, eIF4B: eukaryotic translation initiation factor 4b, ERK: extracellular signal-regulated kinase, FKBP12: FK506 binding protein 12, GβL G protein β subunit like protein, GNAQ: guanine nucleotide-binding protein G(q), HIF: hypoxia-induced factor, mTORC: mTOR complex, NF1: neurofibromin-1, PI3K: phosphoinositide 3-kinase, PKC-a: protein kinase C alpha, PTEN: phosphatase and tensin homolog, RAPTOR: regulatory associated protein of mTOR complex, RHEB: ras homolog enriched in the brain, RICTOR: RPTOR independent companion of mTOR complex 2, Rho: rhodopsin, S6K: S6 kinase, TSC: tuberous sclerosis complex, ULK 1: Unc-51 like autophagy activating kinase, VEGF: vascular endothelial growth factor
Various stimuli like growth factors, cytokines, amino acids, cell stress and mitogens activate the receptor tyrosine kinases resulting in the activation of PI3K, which in turn activates the mTOR-complex 1 through protein kinase B. Activation of mTOR-complex 1 results in angiogenesis, increased translation of protein via S6 kinase and 4E–BP1 and autophagy. Tuberous sclerosis (TS) complex 1 and 2 containing hamartin and tuberin respectively inhibit the phosphorylation of RAS homolog enriched in brain guanosine diphosphate, which inhibits the activation of mTOR-complex 1. Hypoxia, nutrition deprivation and deoxyribonucleic acid damage augment TS complex 1 and 2 and decrease the mTOR-complex 1 activity in normal cells. Protein kinase B and various proto-oncogenes like rat sarcoma virus, rapidly accelerated fibrosarcoma kinase, mitogen-activated protein kinase and extracellular signal-regulated kinase inhibit the TS complex 1 and 2 resulting in mTOR-complex 1 activation. The mTOR-complex 2 is involved in the organisation of actin organelles, cell survival and immune cell differentiation. 2–4
Mammalian target of rapamycin inhibitors
Based on the binding site of mTORi, classified into two generations.
First-generation mTORi are:
-
Rapamycin (sirolimus).
-
Everolimus.
-
Temsirolimus.
-
Ridaforolimus.
The dosage of an oral preparation of sirolimus is 2 mg for adults and 0.8 mg/m2 for children to achieve the target of 5–15 ng/ml in blood. Topical preparation prepared with 0.1–1% concentration compounded with gel, creams and ointment is applied once or twice per day.
Common side effects of oral mTORi are headache, oedema, anaemia, thrombocytopenia, leukopenia, hyperlipidemia, diabetes mellitus, upper respiratory tract infections, insomnia and gastrointestinal disorders. Cutaneous side effects include mucositis, morbilliform exanthema, papulopustular exanthema, nail disorders like paronychia, onycholysis, fragile nails, dystrophy, delayed wound healing, oedema, pruritus, xerosis, alopecia, hypertrichosis and leukocytoclastic vasculitis. 3–5
Second-generation mTORi include a selective mTOR 1/2 inhibitors and dual PI3K/mTORi which are under phase I and II trial. A few examples are INK 128, CC-223 and AZD8055. Second-generation mTORi have broad inhibition of cellular signalling as they target multiple key enzymes and can overcome the known feedback loops occurring with rapalogs.
Mammalian target of rapamycin inhibitors in dermatology
Most of the use in dermatology are off-label and reports are available on genetic, inflammatory, vascular and tumoural origin conditions.
Genodermatoses
Tuberous sclerosis: Autosomal dominant inherited disorder involving TS complex 1 and 2. The mTOR is a promising drug for TS showing significant improvement in subependymal giant cell astrocytoma, renal angiomyolipoma, lymphangiomatosis and reduced frequency of seizure in refractory cases. Focal hypomelanosis and facial angiofibroma, which are resistant to medical treatment, respond well to topical application of mTORi. 6,7
Familial multiple discoid fibromas and Birtt-Hogg-Dube syndrome: It occurs due to mutation in folliculin gene characterised by facial trichofolliculomas. Case reports suggest improvement with topical sirolimus. 8
Pachyonychia congenita: It is an autosomal dominant disorder due to mutation in keratin 6a, 6b, 6c, 16 and 17 characterised by palmoplantar keratoderma, oral leukoplakia, subungual hyperkeratosis and epidermal cysts. Palmoplantar keratoderma responds to topical application of sirolimus. 9
Phosphatase and tensin homolog hamartoma tumour syndrome: This is an autosomal dominant group of disorders with hamartomas in multiple organs due to germline mutation in phosphatase and tensin homolog gene. This includes Cowden syndrome, Lhermitte-Duclos syndrome and Bannayan Riley-Ruvalcaba syndrome. The mTOR inhibitor (oral sirolimus) shows improvement in these as there is the involvement of phosphatase and tensin homolog—PI3K/protein kinase B pathway. 3
Neurofibromatosis type 1: Autosomal dominant condition due to mutation of neurofibromin, which is a suppressor protein of the rat sarcoma virus-mitogen-activated protein kinase-extracellular signal regulated kinase pathway. The mTORi slows the progression of plexiform neurofibromas and improves the pain in neurofibromatosis type 1. 10
Inflammatory/autoimmune conditions
Graft versus host disease (GVHD): GVHD is a common complication following allogeneic stem cell transplantation. mTORi have shown promising results in both acute and chronic GVHD with a response comparable with corticosteroids. 11,12
Lichen planus: Meta-analysis by Andrew et al. showed mixed responses for lichen planus. 13
Beneficial effects of the mTOR inhibitors are reported in other conditions like lupus erythematosus, nephrogenic systemic fibrosis, pemphigus vulgaris, dermatomyositis and eosinophilic fasciitis. 13
Neoplasm
Melanoma: Melanoma cells show vascular endothelial growth factor overexpression and upregulation of phosphoinositide 3-kinase/protein kinase B pathway involving mTOR pathway. Clinical trials show the efficacy and tolerability of the mammalian target of rapamycin inhibitor in combination with bevacizumab or temezolomide or polychemotherapy. Additional combination therapy and monotherapy should be explored. 14
Nonmelanoma skin cancer: mTOR inhibitor is useful in preventing occurrences of nonmelanoma skin cancer, especially in transplant patients, as it inhibits the S6 kinase phosphorylation in the mTOR pathway, which is responsible for tumorigenesis. 4
Kaposi sarcoma: Kaposi sarcoma in transplant patients is increasing due to immunosuppression. The mTOR inhibitor (oral sirolimus) showed excellent response both in the skin and visceral Kaposi sarcoma and now it is considered the drug of choice for the same. 4
Cutaneous T cell lymphoma: Excessive activation of the mTOR pathway is found in sezary syndrome and other cutaneous T cell lymphoma. Clinical trial shows improvement with oral mTOR inhibitor. Topical mTOR inhibitor treatment for the same is being explored. 4
Vascular anomalies and neoplasms: Most of the vascular anomalies are due to mutation in the TIE2/receptor tyrosine kinases, guanine nucleotide-binding protein/11, rat sarcoma virus, rapidly accelerated fibrosarcoma kinase, mitogen activated protein kinase, extracellular signal-regulated kinase and SMAD4 which is interlinked with mTOR pathway downstream. mTOR inhibits angiogenesis by reducing vascular endothelial growth factor, decreasing the response of endothelial cells to vascular endothelial growth factor signalling, and downregulating the protein kinase B pathways. Vascular anomalies that are responsive to the mammalian target of rapamycin inhibitor are listed in Table 1.
|
Ageing: Topical application of rapamycin reduces cellular senescence, which is consistent with the expression of P16INK4A. Christina et al. showed improvement in clinical skin appearance and histological markers of ageing with an increase in collagen VII in the basement membrane, indicating the topical mTOR inhibitor as a potential anti-ageing therapy. 15
Overall mTORi uses and common side effects are mentioned in Table 2.
| Usage | Common side effects |
|---|---|
|
Sirolimus FDA approved: prophylaxis against renal transplant rejection |
|
|
Everolimus FDA approved: —
|
|
|
Temsirolimus FDA approved:
|
|
|
Non-FDA approved—usage of various mammalian targets of rapamycin inhibitor: Genodermatoses:
Autoimmune/Inflammatory conditions:
Neoplasms:
Miscellaneous:
|
Conclusion
The pathogenic relationship of the mTOR pathway in many dermatological conditions has been studied in the last 2 decades and it has shown promise in many difficult-to-treat dermatological disorders. More research in the form of multiple randomised clinical trials, safety and efficacy of both oral and topical preparation of mTOR inhibitor, direct comparison of new mTOR inhibitor with existing ones and development of new mTOR inhibitors are required.
Declaration of patient consent
Patient consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Discoverer of the treasure from a barren island: Suren Sehgal (10 February 1932 to 21 January 2003) Transplantation. 2003;76:623-4.
- [Google Scholar]
- Elucidation of the mTOR pathway and therapeutic applications in dermatology. Actas Dermosifiliogr. 2016;107:379-90.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in the therapeutic use of mammalian target of rapamycin (mTOR) inhibitors in dermatology. J Am Acad Dermatol. 2015;72:879-89.
- [CrossRef] [PubMed] [Google Scholar]
- m-TOR inhibitors and their potential role in haematological malignancies. Br J Haematol. 2017;177:684-702.
- [CrossRef] [PubMed] [Google Scholar]
- Everolimus in the treatment of neuroendocrine tumors: efficacy, side-effects, resistance, and factors affecting its place in the treatment sequence. Expert Opinion on Pharmacotherapy. 2018;19:909-28.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molecular genetics of the PI3K-AKT-mTOR pathway in genodermatoses: Diagnostic implications and treatment opportunities. J Invest Dermatol. 2016;136:15-23.
- [CrossRef] [PubMed] [Google Scholar]
- Perfect match: mTOR inhibitors and tuberous sclerosis complex. Orphanet J Rare Dis. 2022;17:106.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Topical Rapamycin as a Treatment for Fibrofolliculomas in Birt-Hogg-Dubé Syndrome: A Double-Blind Placebo-Controlled Randomized Split-Face Trial. PLoS ONE. 2014;9:e99071.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Novel treatment of painful plantar keratoderma in pachyonychia congenita using topical sirolimus. Clin Exp Dermatol 2018:968-71.
- [CrossRef] [PubMed] [Google Scholar]
- Neurofibromatosis type 1: New developments in genetics and treatment. J Am Acad Dermatol. 2021;84:1667-76.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized multicenter trial of sirolimus vs prednisone as initial therapy for standard-risk acute GVHD: the BMT CTN 1501 trial. Blood. 2020;135:97-107.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-96.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review of sirolimus in dermatological conditions. Australas J Dermatol. 2021;62:461-9.
- [CrossRef] [PubMed] [Google Scholar]
- mTOR inhibitor everolimus reduces invasiveness of melanoma cells. Human Cell. 2020;33:88-97.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Topical rapamycin reduces markers of senescence and aging in human skin: an exploratory, prospective, randomized trial. Geroscience. 2019;41:861-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





