Translate this page into:
Melanoma arising in a giant congenital melanocytic nevus with neuroblastoma RAS mutation
Corresponding author: Dr. Neetu Bhari, Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi - 110 029, India. drntbhari@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dudani P, Senthilnathan G, Wajid MA, Gupta AK, Kaushal S, Arora S, et al. Melanoma arising in a giant congenital melanocytic nevus with neuroblastoma RAS mutation. Indian J Dermatol Venereol Leprol 2021;87:416-20.
Sir,
Malignant melanoma is uncommon in Asian population and pre-pubertal incidence is extremely rare. Pediatric melanoma is not a congener of adult melanoma with varying risk factors, morphology, behavior and prognosis.1 Congenital melanocytic nevi are recognized risk factors for melanoma, directly proportional to their number.2
A four-year-old girl presented with a rapidly growing hyperpigmented nodule on her back, within her large congenital melanocytic nevus, for the past four months. It was extremely tender on palpation and associated with occasional bleeding following minor trauma. There were no other systemic complaints and family history was unremarkable.
Clinical examination revealed a single, large melanocytic nevus (>20 cm diameter) on her back (Fitzpatrick type 3 skin), along with multiple satellite nevi sized 0.5–8 cm. The overlying nodule (8 cm x 7 cm x 4 cm) was sessile, lobulated and erythematous to variegate hyperpigmented [Figure 1]. We noted focal areas of ulceration and superficial hemorrhages on its surface. Both nodule and surrounding skin were distinctly tender. There was no clinical lymphadenopathy or hepatosplenomegaly. Dermoscopy of the nodule showed lobulated surface with peripheral linear and curved vessels, ulceration, blue white veils, depigmented areas and yellow-orange homogenous areas with yellow-brown crusting [Figure 2].
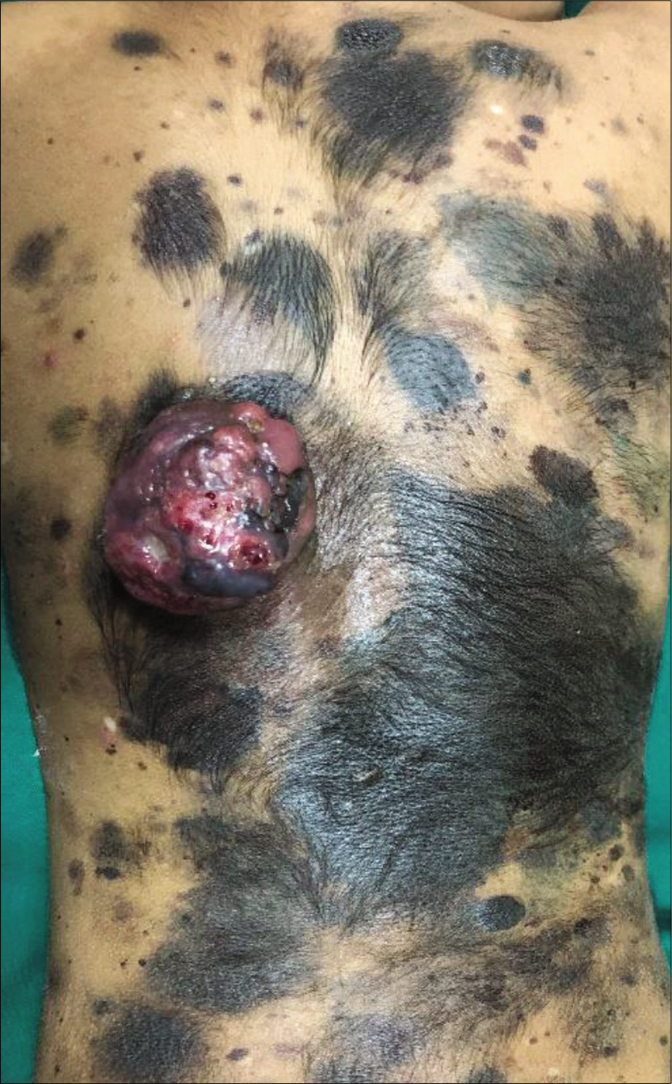
- Ulcerated lobulated nodule of size 8 cm × 7 cm × 4 cm present over congenital melanocytic nevus of size 20 cm over the back with multiple satellite nevi of size 0.5–8 cm
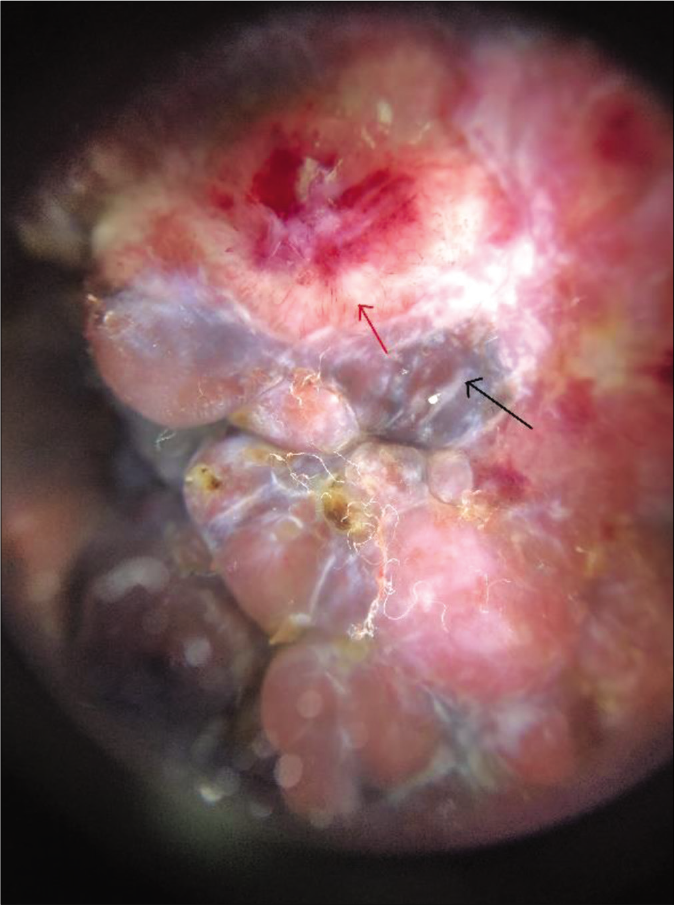
- Dermoscopic analysis of the nodule (DermLite 4, polarized, 10x, magnified) shows lobulated surface with surface linear and curved vessels (red arrow), ulceration, blue-white veils (black arrow), depigmented areas and yellow-orange homogenous areas with yellow-brown crusting
Biopsy of the nodule revealed invasive neoplastic cells arranged in sheets. These cells contained abundant eosinophilic cytoplasm, round to oval nuclei with nuclear pleomorphism, prominent nucleoli, occasional nuclear pseudo-inclusions and multiple atypical mitoses; diagnostic of malignant melanoma [Figures 3a and b]. Immunohistochemistry showed diffuse cytoplasmic positivity for HMB 45 (anti-gp100) and Melan-A [Figures 3c and d]. Positron emission tomography showed lesional uptake along with a small adjacent focus and solitary left axillary lymph node [Figures 4a and b]. Excision biopsy from the latter also showed tumor deposits [Figure 5a].
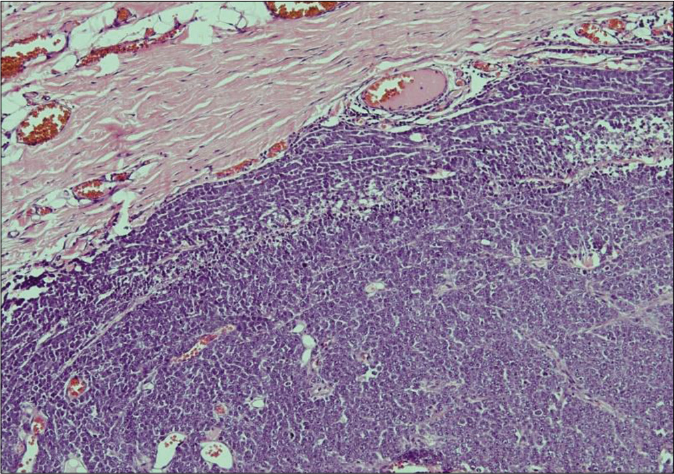
- Histopathology of the nodule shows an invasive tumor predominantly arranged in sheets (H and E, ×100)
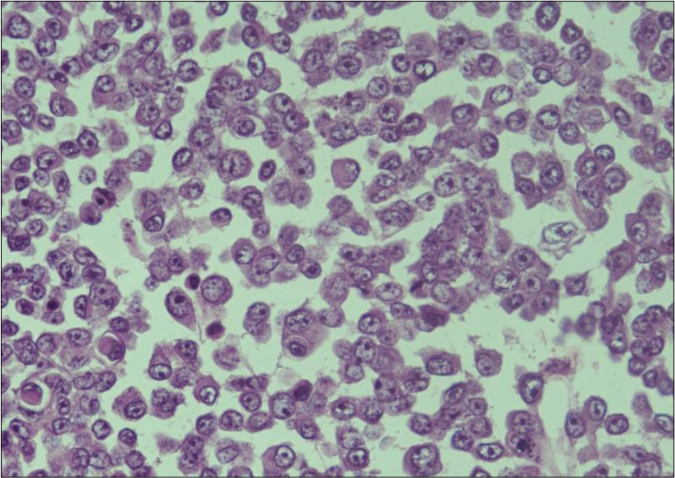
- Higher magnification shows melanoma cells with abundant eosinophilic cytoplasm, prominent nucleoli and occasional nuclear pseudoinclusions (H and E, ×400)
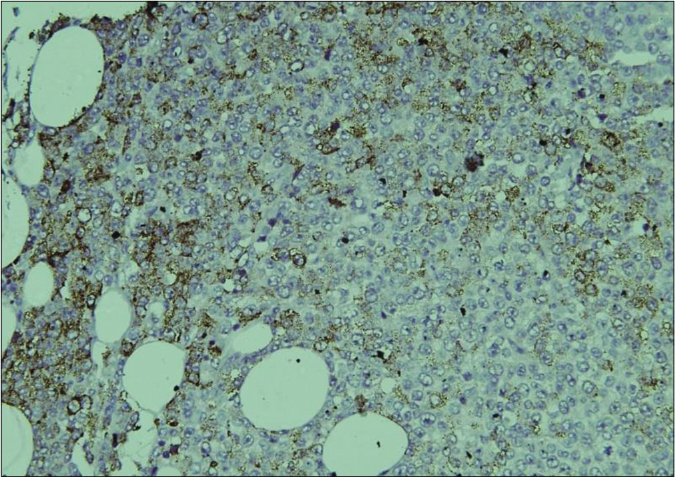
- On immunohistochemistry, the tumor cells show diffuse cytoplasmic positivity for HMB 45 (IHC, DAB chromogen, ×200)
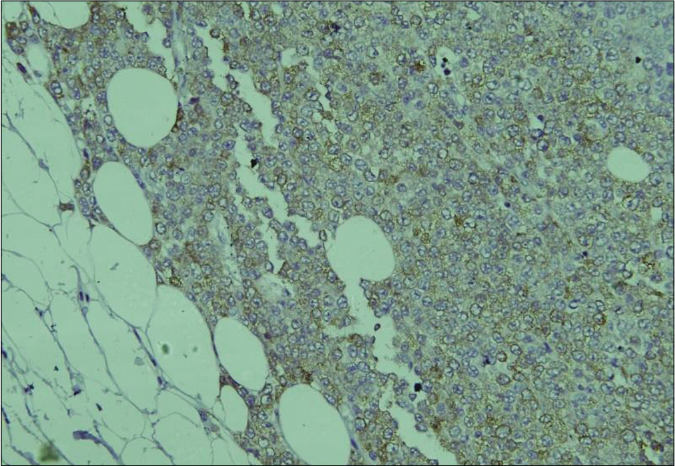
- On immunohistochemistry, the tumor cells show diffuse cytoplasmic positivity for Melan A (IHC, DAB chromogen, ×200)
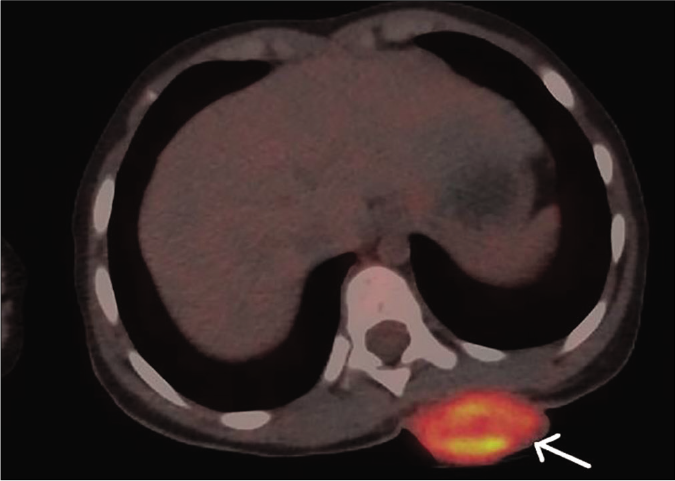
- Maximum intensity projection of fused 18F-fluorodeoxyglucose positron emission tomography/computed tomography images showing heterogeneous mass measuring 2.5 cm × 4.4 cm in the left posterior chest wall with increased fluorodeoxyglucose uptake (maximum standardized uptake value – 73.9) (white arrow)
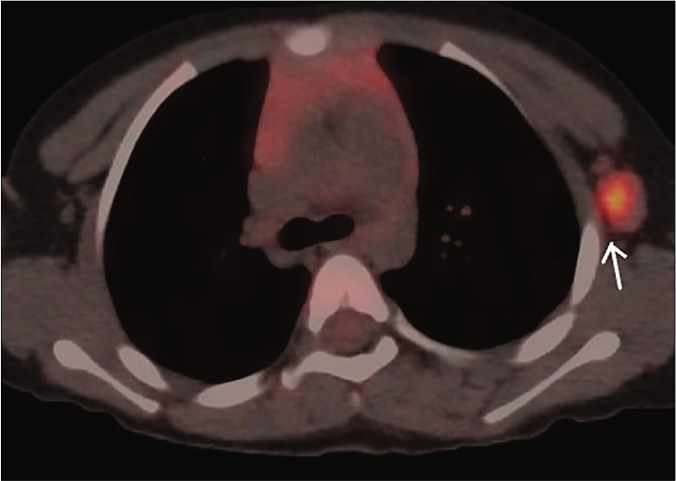
- Maximum intensity projection, of fused 18F-fluorodeoxyglucose positron emission tomography/computed tomography images showing enlarged left axillary lymph node measuring 1.8 cm × 1.2 cm with increased FDG uptake (maximum standardized uptake value – 42.1) (white arrow). *Standardized uptake value
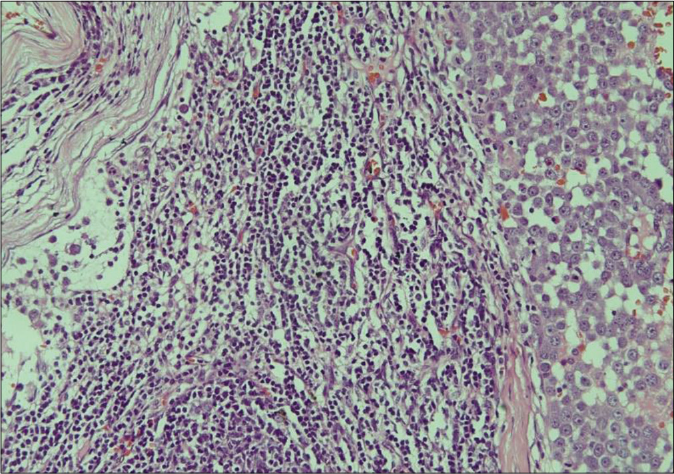
- Histopathology of the excised lymph node showing proliferation of atypical melanocytic cells in the dermis (H and E, ×200)
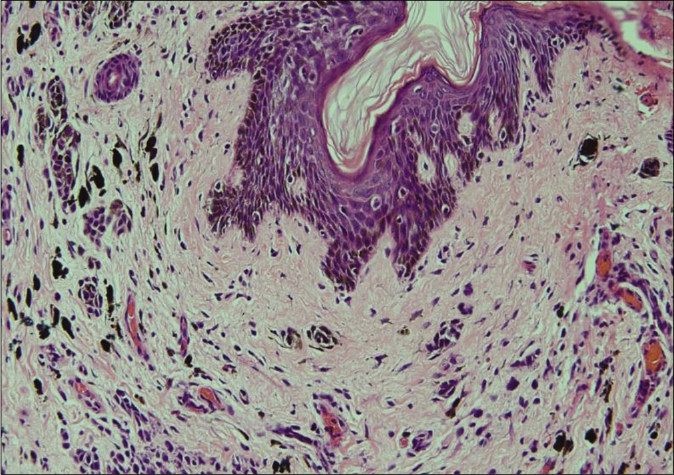
- Margin of the excised nodule shows a dermal nevus with melanin pigment (H and E, ×400)
Magnetic resonance imaging of brain was unremarkable. Her disease was Stage III, as per American Joint Committee on Cancer staging. Genetic mutational analysis revealed a missense mutation in neuroblastoma RAS viral oncogene (NRAS) gene (chr1:115256529:T>A:c.182A>T, p.Gln61Leu/ Exon3). No mutations were detected in proto-oncogene B-Raf (BRAF) and c-kit (receptor tyrosine kinase) genes.
The nodule was surgically excised along with a 1 cm margin. Histopathology of excised specimen showed tumor-free margins [Figure 5b]. We administered an induction therapy of 20 million IU/m2, interferon alpha 2b, five days per week for four weeks. Maintenance therapy consisted of 10 million IU per m2, interferon alpha 2b, three times per week along with injection nivolumab (3 mg/kg) once in four weeks for three cycles (12 weeks). Thereafter, we advised palliative care as new lymph nodes became palpable suggestive of disease relapse. She survived for 4 months after discontinuing chemotherapy.
Giant congenital melanocytic nevus is characterized by a diameter exceeding 20 cm by adulthood and is associated with 5-40% life-time risk of developing melanoma at the lesional site, most occurring within the first 5 years of life.3,4 Neuromelanosis and melanoma are more likely with size >40 cm, number >20 or multiple satellite lesions and a truncal location. A gadolinium-enhanced screening magnetic resonance imaging (MRI) of the brain and whole spine is recommended <1 year age in a child presenting with this condition.4,5 If found normal, annual neurodevelopmental monitoring should be done until school age for early detection of developmental aberrations such as speech delay. Any abnormality on MRI should prompt a regular clinical and radiological evaluation. Family members of such a child should be educated about regular self-examination of skin and to report any new lesion, secondary surface change or rapid growth of existing lesion. This reported lesion should be observed for four weeks with baseline photographs. In case of progression or development of other abnormalities like lymphadenopathy, excision biopsy should be performed.
There is no accepted standard treatment for this condition, and surgical excision remains controversial. Surgery fails to address the risk of extracutaneous melanoma, however, removal of melanocytic cells may reduce risk of developing melanoma within the nevus. Flaps or pigmented excised epidermis may be used for reconstruction following staged surgical excision. Alternate treatment options include combinations lasers (diode, fractional carbon dioxide and q-switched Nd-YAG lasers) and curettage.4,6,7
Compared to adults, pediatric melanomas have more atypical features, thicker lesions at diagnosis, higher frequency of nodular histotype and more aggressive course [Table 1].1 A mutational analysis concluded that sun-induced pediatric melanomas exhibit a UV-induced mutational spectrum similar to its adult counterpart (telomerase reverse transcriptase-promoter (TERT-p), BRAF V600 and phosphatase and tensin homolog (PTEN) mutations). NRAS mutation is more frequent in melanomas arising within congenital melanocytic nevi,8 also seen in our patient.
| Adult | Pediatric | |
|---|---|---|
| Site | Typical – photoexposed, acral | Lower limbs |
| Etiology | Sun exposure | Sun exposure for sporadic, genetic predisposition for familial cases and those arising within CMN |
| Mutation | BRAF V600 (60%) NRAS (20%) |
Sporadic (BRAF V600, PTEN) Familial (CDKN2A, CDK4) CMN-associated: NRAS, PTEN |
| Diagnostic features | ABCDE (sensitivity 58%–76%) | ABCDE misleading, >50% amelanotic |
| Thickness at presentation | Less | More |
| Progression | Slow | Rapid |
| Lymph node metastasis | Later | Earlier, in 50%–70% of borderline lesions |
| Prognosis | Poor | Relatively better (age<10 years) |
ABCDE: Asymmetry, border irregularity, color variation, diameter greater than 6 mm and evolving size, shape or color. BRAF: Proto-oncogene B-Raf, NRAS: Neuroblastoma RAS viral oncogene, PTEN: Phosphatase and tensin homolog, CDKN2A: Cyclin-dependent kinase inhibitor 2A, CDK4: Cyclin-dependent kinase 4, CMN: Congenital melanocytic nevi
On dermoscopy of the nodule, we observed blue-white veil, linear peripheral vessels and depigmented areas; suggestive of nodular melanoma.9,10 Multiple authors have reported a more aggressive course and sentinel lymph nodal metastases in young children as compared to adults, however, their survival rate remains better than post-pubertal and adult patients.3 Our patient also showed a rapid progression and lymph node involvement. Specific guidelines are lacking concerning management of melanoma arising in a congenital melanocytic nevus. Lesional surgical excision with adjuvant chemotherapy remains the treatment of choice. Choice of adjuvant in children is based on adult studies, as data are scarce in the former group. Nivolumab was approved by the US Food and Drug Administration (FDA) in December 2017 for adjuvant therapy of cutaneous melanoma with lymph node metastasis and Stage IV disease post-definitive resection of lesion(s). Other adjuvants used for this indication include pembrolizumab, ipilimumab and interferon alpha. We selected nivolumab for its ability to prolong relapse-free survival with reduced systemic toxicity compared to ipilimumab, in patients with resected stage III/IV melanoma at high risk of recurrence.11
Acknowledgment
We thank Dr. S.V.S. Deo and his team at Dr. B. R. Ambedkar Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, Delhi, for the surgical excision of tumor.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Does melanoma behave differently in younger children than in adults? A retrospective study of 33 cases of childhood melanoma from a single institution. Pediatrics. 2005;115:649-54.
- [CrossRef] [PubMed] [Google Scholar]
- Melanoma in children and adolescents. Diagnostic Histopathol. 2008;14:18-27.
- [CrossRef] [Google Scholar]
- Congenital melanocytic nevi: Clinical and histopathologic features, risk of melanoma, and clinical management. J Am Acad Dermatol. 2005;52:197-203.
- [CrossRef] [PubMed] [Google Scholar]
- Melanoma arising in a Giant congenital melanocytic nevus: Two case reports. Diagn Pathol. 2019;14:21.
- [CrossRef] [PubMed] [Google Scholar]
- Melanoma in congenital melanocytic naevi. Br J Dermatol. 2017;176:1131-43.
- [CrossRef] [PubMed] [Google Scholar]
- Giant congenital melanocytic nevi successfully treated with combined laser therapy. Indian Dermatol Online J. 2020;11:79-82.
- [Google Scholar]
- Curettage of giant congenital melanocytic nevi in neonates: A decade later. Arch Dermatol. 2002;138:943-7.
- [CrossRef] [PubMed] [Google Scholar]
- The genomic landscape of childhood and adolescent melanoma. J Invest Dermatol. 2015;135:816-23.
- [CrossRef] [PubMed] [Google Scholar]
- Melanoma in the young: Differences and similarities with adult melanoma: A case-matched controlled analysis. Cancer. 2007;110:614-24.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopic evaluation of nodular melanoma. JAMA Dermatol. 2013;149:699-709.
- [CrossRef] [PubMed] [Google Scholar]
- Adjuvant nivolumab versus ipilimumab in resected Stage III or IV melanoma. N Engl J Med. 2017;377:1824-35.
- [CrossRef] [PubMed] [Google Scholar]





