Translate this page into:
Morphea: Evidence-based recommendations for treatment
Correspondence Address:
Nicole M Fett
Department of Dermatology, Perelman Center for Advanced Medicine, University of Pennsylvania, Suite 1-330A, 3400 Civic Center Boulevard, Philadelphia, PA 19104
USA
| How to cite this article: Fett NM. Morphea: Evidence-based recommendations for treatment. Indian J Dermatol Venereol Leprol 2012;78:135-141 |
Abstract
Morphea is a rare fibrosing disorder of the skin. Evidence-based treatment strategies in morphea are lacking. This review summarizes the available data on morphea treatment and provides therapeutic strategies based on morphea subtypes. The Cochrane Library, Medline and Embase from inception until May of 2011 were searched using the key words "morphea" and "morphea treatment." Reference lists of the resultant articles, as well as relevant reviews, were also searched. This review focuses on randomized controlled trials, prospective interventional trials without controls and retrospective reviews with greater than five subjects.Introduction
Morphea is a rare fibrosing disorder of the skin that may also involve the underlying muscle, connective tissue, bone and brain. The pathogenesis of morphea is incompletely understood, but results in an increase of collagen production and decrease in collagen destruction. Morphea typically goes through two stages: an active (inflammatory) stage and a "burnt out" stage. Treatment is targeted at the active phase, in the hope of stabilizing the size of current lesions and preventing the occurrence of new lesions. Anti-inflammatory and immunosuppressive agents are unlikely to improve the burnt-out phase of morphea and, therefore, should not be used for this phase of the disease. Treatment algorithms for morphea are lacking due to the relative paucity of treatment data. This article will review the strongest data in morphea treatment (randomized controlled trials, prospective trials and retrospective data with greater than five subjects) [Table - 1]. Recommended treatment algorithms are provided for the following subsets of morphea: circumscribed morphea [Figure - 1], linear morphea affecting the head or limbs [Figure - 2] and generalized morphea (defined as four or more indurated plaques larger than 3 cm involving two or more body sites) [Figure - 3].
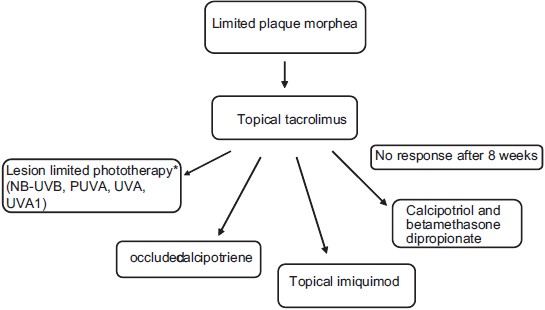 |
| Figure 1: Treatment algorithm for limited plaque morphea. (Figure adapted from Fett N and Werth VP. Update on morphea. JAAD. 2011;64:217-242[28]), *Based on local availability |
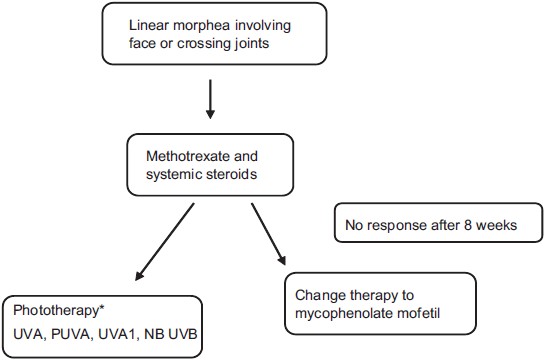 |
| Figure 2: Treatment algorithm for linear morphea involving the face or crossing joints. (Figure adapted from Fett N and Werth VP. Update on morphea. JAAD. 2011;64:217-242[28]), *Based on local availability |
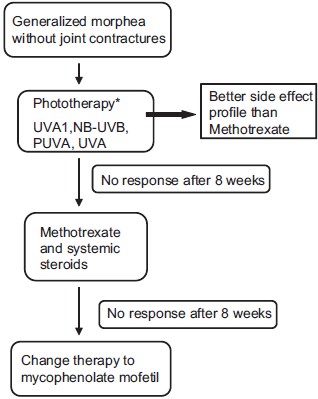 |
| Figure 3: Treatment algorithm for generalized morphea. (Figure adapted from Fett N and Werth VP. Update on morphea. JAAD. 2011;64:217-242[28]), *Based on local availability |
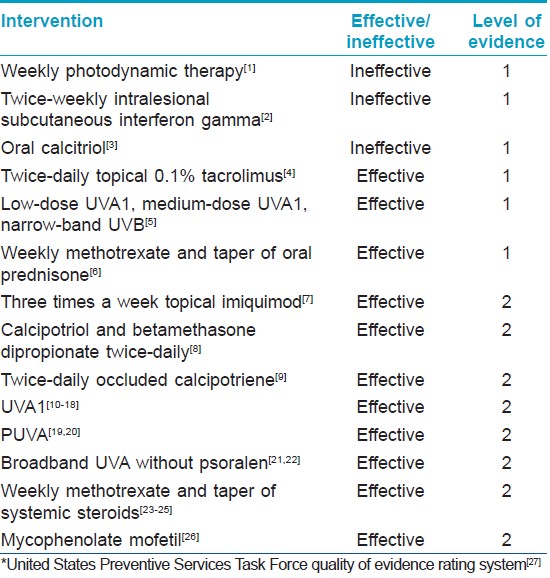
Trials of Topical Treatment
Randomized placebo-controlled trials of topical therapy
Two randomized placebo-controlled trials of topical therapy have been conducted. Kroft et al. assessed the efficacy of topical 0.1% tacrolimus in the treatment of plaque morphea. [4] Ten subjects were enrolled. Each subject had two or more active plaques of morphea, thereby serving as their own control. The plaques were separated by at least 15 cm. Subjects applied tacrolimus to one of the lesions and vehicle to the other. The primary outcome measures were change in surface area, change in durometer score (an assessment of skin hardness) and change in an investigator-derived clinical feature score made up of dyspigmentation, induration, erythema, telangiectasia and atrophy scales. [4] Plaques treated with topical tacrolimus had statistically significant reductions in both durometer scores and clinical feature scores when compared with placebo. [4] This suggests that topical tacrolimus is an effective treatment for an active (inflammatory) plaque morphea.
Batchelor et al. performed a randomized lesion-controlled trial of photodynamic therapy (PDT) in the treatment of morphea. [1] Seven subjects were enrolled and six subjects completed all treatments. After 6 weeks of weekly PDT treatments, no significant change was noted between treated and untreated lesions. [1] This suggests that PDT is not an effective therapy for an active plaque morphea.
Prospective pilot studies of topical therapy
The efficacy of thrice-weekly imiquimod in the treatment of morphea was assessed in a prospective pilot study of 12 subjects. [7] The primary outcome measure was an author-derived clinical score including dyspigmentation, induration, erythema and telangiectasias scales. [7] There was a statistically significant decrease in erythema and induration scores, both reflective of disease activity, at 6 months. [7] Dyspigmentation and telangiectasias scores, both reflective of damage, did not decrease over time. [7] This study is limited by the lack of a placebo group and by its long duration. It would not be unexpected for active plaques of morphea to burn out without intervention after a 6-month time lapse.
The efficacy of calcipotriol in combination with betamethasone dipropionate in the treatment of plaque morphea was assessed in a prospective pilot study of six subjects. Subjects were instructed to apply the combination calcipotriol and betamethasone dipropionate once or twice a day to the active lesion. The authors reported an improvement in five of six subjects. [8] This study is limited by the lack of a placebo group. The results suggest that the combination of calcipotriol and betamethasone dipropionate once to twice daily may be an effective therapy for active plaque morphea.
Lastly, the efficacy of twice-daily occluded topical calcipotriene in the treatment of active plaque or linear morphea was assessed in a prospective pilot study of 12 subjects. The primary outcome measure was an author-derived skin score. Subjects were treated for a duration of 3 months. Twice-daily occluded calcipotriene resulted in a statistically significant decrease in erythema, dyspigmentation, telangiectasia and induration. [9] This study is limited by the lack of placebo. This study suggests that twice-daily occluded calcipotriene is an effective therapy for active plaque or linear morphea.
Trials of Systemic Treatment
Randomized controlled trials of systemic therapy
Two negative randomized placebo-controlled trials have been conducted: the assessment of subcutaneous intralesional interferon gamma in the treatment of morphea and oral calcitriol. The efficacy of subcutaneous intralesional interferon gamma in the treatment of active plaque morphea was assessed by Hunzelmann et al. [2] Twenty-four subjects were randomized to receive either 14 doses of interferon gamma over 6 weeks, or placebo. [2] The primary outcome measures were a change in skin surface area, change in an author-derived skin score and change in the number of lesions. [2] There was no statistically significant change between the interferon-treated group and the placebo group at 18 weeks. [2] This suggests that interferon gamma is not an effective therapy for morphea.
The second negative study, published in 2000, evaluated the effectiveness of oral calcitriol as a therapy for morphea. [3] Twenty subjects were randomized to oral calcitriol or placebo for 9 months. [3] The primary outcome measure was an author-derived skin score. The placebo group had a greater decrease in their skin scores than the calcitriol group. [7] This suggests that oral calcitriol is not an effective treatment for morphea.
Two positive randomized controlled trials assessing morphea therapy have been published.
The first, published in 2006, randomized 62 subjects to receive low-dose ultraviolet A1 (UVA1; 340-400 nm), medium-dose UVA1 and narrow-band ultraviolet B (NB-UVB) therapy for morphea. [5] Subjects were treated for eight consecutive weeks. The UVA1group received a total dose of 800 J/cm 2 , the medium-dose UVA1 group was given a total dose of 2000 J/cm 2 and the narrow-band UVB group was started at 0.1 J/cm 2 for skin type 2 and 0.2 J/cm 2 for skin type 3 and was increased by 0.1-0.2 J/cm 2 as tolerated, with maximum doses of 1.3 J/cm 2 for skin type 2 and 1.5 J/cm 2 for skin type 3. [5] Outcome measures were an author-derived skin score (the modified skin score, or MSS), scores on a visual analog scale, changes in the histological appearance and 20 MHz ultrasound measurements. All three groups had a statistically significant decrease in skin scores. [5] Comparison of the skin score changes between treatment arms revealed that the medium-dose UVA1 group showed statistically significant improvements in skin score compared with the NB-UVB, but was equivalent to the low-dose UVA1 group. [5] Low-dose UVA1 and NB-UVB groups also had equivalent improvement in skin scores. [5] This study is limited by the lack of a placebo group, but suggests that low-dose UVA1, medium-dose UVA1 and narrow-band UVB may all be effective therapies for morphea.
The second trial published in 2011 is a double-blind randomized study of combination of 3 months of 1 mg/kg/day oral prednisone and 1 year of weekly oral methotrexate compared with 3 months of oral 1 mg/kg/day prednisone in children with active linear, generalized or mixed variant morphea. [6] Seventy subjects were randomized in a two to one fashion, resulting in 46 subjects treated with prednisone and methotrexate and 24 subjects treated with prednisone alone. [6] The outcome measures were time to disease relapse and comparison of "responders" in each group. [6] Responders were defined as children who had a skin surface ratio of less than one (this proportion accounts for the normal growth of the children), a 10% decrease in thermography measurement and no new lesions. [6] There was a statistically significant prolongation in time to relapse in the methotrexate-treated group, suggesting that the combination of oral prednisone and methotrexate is an effective therapy for active morphea.
Prospective trials of systemic therapy
Three prospective trials of the therapeutic effects of methotrexate in combination with systemic corticosteroids in morphea have been performed. [23],[24],[25] These studies assessed the response of morphea to methotrexate treatment in a total of 24 adults and 10 children. Adults were treated with 15 mg of methotrexate a week, with doses adjusted based on clinical response. Children were treated with 0.3 mg/kg per week, with doses adjusted to response as well. All subjects received bursts of high-dose intravenous methylprednisolone as well. The outcome measures in the adult studies were change in mean skin score and change in mean ultrasound thickness. [23],[24] Both adult studies revealed a statistically significant decrease in mean skin score and mean ultrasound measurements when compared with baseline. [23],[24] Nine of the 10 children were reported to improve based on clinician assessment. [25] These three prospective studies suggest that treatment of morphea with systemic methotrexate and steroids is effective.
Since 1995, 121 subjects with morphea have been prospectively treated with UVA1. [10],[11],[12],[13],[14],[15],[16],[17],[18] Subject ages ranged from 3 to 73 years and disease duration ranged from 6 months to 20 years. Subjects with linear, plaque and deep morphea were included in these studies. Seventy of the 121 subjects were treated with low-dose UVA1, at doses of 20 J/cm 2 /day tapered over 5-20 weeks, with total irradiation ranging from 600 to 800 J/cm 2 . Of these 70 subjects treated with low-dose UVA1, 90% reportedly improved based on changes in clinical examination, an author-derived skin score, changes in ultrasound measurement, changes in cutometer measurements, skin biopsies or a combination of these outcome measures. [10],[11],[13],[14],[15],[17] Two studies compared medium-dose UVA1 (70 J/cm 2 /treatment with a total dose of 2100 J/cm 2 ) and high-dose UVA1 (130 J/cm 2 /treatment with a total dose of 3900 J/cm 2 ) to low-dose UVA1 (20 J/cm 2 /treatment with a total dose of 600 J/cm2). [12],[15] All three treatment modalities showed improvement in outcome measures, with suggestion that the duration of improvement was more prolonged with higher doses. [12],[15]
Thirty subjects with morphea have been prospectively treated with psoralen in combination with UVA (PUVA). [19],[20] Of these 30 subjects, 80% had improvement in skin scores. [19],[20]
Seventy-five subjects with morphea have been prospectively treated with broadband UVA without psoralen. [21],[22] Broadband UVA doses ranged from 5 J/cm 2 /treatment with a total of 100 J/cm 2 of irradiation to 20 J/cm 2 /treatment with a total of 400 J/cm 2 of irradiation, with equivalent improvements in both groups. [22] Of these 75 subjects, 77% were reported to have "fair" or better response to therapy based on clinical assessment. [21],[22] These studies suggest that broadband UVA therapy and PUVA may be effective treatments for morphea.
Retrospective trials of systemic therapy
In the last 4 years, four retrospective reviews on the use of methotrexate have been carried out on a total of 119 patients with morphea. [29],[30],[31],[32] Of these 119 patients, 67 received methotrexate in combination with systemic corticosteroids, and 52 received methotrexate without systemic steroids. [29],[30],[31],[32] Methotrexate doses ranged from 0.3 to 0.4 mg/kg/week in children and 15 to 25 mg/week in adults. [29],[30],[31],[32] Systemic corticosteroids were given via intravenous pulse and then transitioned to oral. Seventy-nine percent of the 119 patients reportedly improved with treatment. [29],[30],[31],[32]
Mycophenolate mofetil (MMF) has been retrospectively assessed as a treatment for pansclerotic, generalized, linear and mixed variant morphea in children. [26] All these subjects had failed to improve with corticosteroids, methotrexate or a combination of the two. [26] The primary outcome measures were clinician assessment of improvement and change in thermography. Nine of 10 patients reportedly improved with MMF therapy. [26] This study is limited by its retrospective nature and lack of a placebo group. MMF has been shown to have anti-proliferative and anti-fibrotic properties in in vitro and in vivo experiments. [33],[34],[35],[36],[37],[38],[39] MMF therapy has resulted in a statistically significant improvement in skin scores in subjects with diffuse systemic sclerosis and improvement in retroperitoneal fibrosis. [36],[39]
Recommended Treatment Algorithms
Before making decisions about treatment options, patients with morphea need to be counseled on the prognosis of their disease process. It is important for patients to enter into therapy knowing that morphea is not life-threatening and does not progress to systemic sclerosis. Patients with linear morphea are at risk for facial deformity, limb length discrepancy and contractures. Patients need to balance the risks of systemic therapy with the risk of untreated disease. Patients with plaque morphea and superficial generalized variants will generally be left with hyperpigmentation as the only sign of prior disease. Patient′s expectations should also be managed. Patients need to recognize that the involved skin will never look completely normal. They must be counseled that treatment is aimed at active disease in the hopes of preventing enlargement of already-present lesions and the development of new lesions. Burnt-out disease is unlikely to improve with immunosuppression and, therefore, the risks of these medications are not warranted in burnt-out disease.
Patients with limited plaque morphea are at very low risk of facial deformity, limb length discrepancy and contractures and therefore should be treated with topical agents whenever possible. Based on the available data, active limited plaque morphea should be primarily treated with topical tacrolimus twice daily. If no response is seen after 8 weeks, therapy may be changed to lesion limited-phototherapy (NB-UVB, BB-UVA, UVA1, or topical psoralen and UVA), twice-daily occluded calipotriene, a combination of calcipotriol and betamethasone dipropionate once to twice a day or thrice-weekly topical imiquimod. Topical steroids, the most commonly utilized therapy for active limited plaque morphea, may be effective but, to date, we do not have data supporting their efficacy.
Patients with linear morphea of the head and neck or limbs are at significant risk of facial deformity, limb length discrepancy and contractures and should, therefore, be treated with systemic therapy. Based on the available evidence, methotrexate in combination with a short course of systemic steroids is first-line therapy. If the patient does not show improvement after 2-3 months, therapy can be switched to phototherapy (NB-UVB, PUVA, UVA or UVA1) based on availability. If phototherapy is not available or impossible for the patient because of the time commitment required, I recommend a trial of MMF. The evidence for MMF in the treatment of morphea is weak; however, it is not weaker than the evidence for the use of other systemic immunosuppressives such as cyclosporine, imatinib, d-penicillamine, cyclosphosphamide, tumor necrosis factor-alfa inhibitors or extracorporal photopheresis, [40],[41],[42],[43],[44],[45],[46] and it has a more favorable side-effect profile than these treatment modalities. MMF may also have anti-fibrotic properties based on the in vitro and in vivo studies discussed above. It is with consideration of the data, MMF′s more favorable side-effect profile and anti-fibrotic properties that I recommend it in patients with potentially deforming disease who fail methotrexate and steroids.
Patients with generalized morphea may have superficial or deep variants. In patients without lesions that cross joints, phototherapy is an appropriate first option. At this point, there is not enough data to recommend one type of phototherapy over another. There is data supporting the use of NB-UVB, PUVA, UVA and UVA1. Phototherapy has a more favorable side-effect profile than methotrexate and systemic steroids, which is why I favor it as a primary therapy in this subset of patients. If the patient has not improved after 2-3 months, then switching therapy to methotrexate in combination with systemic steroids is a next step. If, after an additional 2-3 months the patient has not improved, I recommend a trial of MMF.
In conclusion, additional trials of therapeutic options for patients with morphea are needed. Randomized trials focusing on comparison of the efficacy of different types of phototherapy with the addition of a placebo group would be particularly useful. Randomized placebo-controlled trials assessing additional systemic agents in those patients recalcitrant to methotrexate in combination with steroids are also necessary.
| 1. |
Batchelor R, Lamb S, Goulden V, Stables G, Goodfield M, Merchant W. Photodynamic therapy for the treatment of morphoea. Clin Exp Dermatol 2008;33:661-3.
[Google Scholar]
|
| 2. |
Hunzelmann N, Anders S, Fierlbeck G, Hein R, Herrmann K, Albrecht M, et al. Double-blind, placebo-controlled study of intralesional interferon gamma for the treatment of localized scleroderma. J Am Acad Dermatol 1997;36:433-5.
[Google Scholar]
|
| 3. |
Hulshof MM, Bouwes Bavinck JN, Bergman W, Masclee AA, Heickendorff L, Breedveld FC, et al. Double-blind, placebo-controlled study of oral calcitriol for the treatment of localized and systemic scleroderma. J Am Acad Dermatol 2000;43:1017-23.
[Google Scholar]
|
| 4. |
Kroft EB, Groeneveld TJ, Seyger MM, de Jong EM. Efficacy of topical tacrolimus 0.1% in active plaque morphea: Randomized, double-blind, emollient-controlled pilot study. Am J Clin Dermatol 2009;10:181-7.
[Google Scholar]
|
| 5. |
Kreuter A, Hyun J, Stucker M, Sommer A, Altmeyer P, Gambichler T. A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. J Am Acad Dermatol 2006;54:440-7.
[Google Scholar]
|
| 6. |
Zulian F, Martini G, Vallongo C, Vittadello F, Falcini F, Patrizi A, et al. Methotrexate in juvenile localized scleroderma: A randomised, double-blind, placebo-controlled trial. Arthritis Rheum 2011;63:1998-2006.
[Google Scholar]
|
| 7. |
Dytoc M, Ting PT, Man J, Sawyer D, Fiorillo L. First case series on the use of imiquimod for morphoea. Br J Dermatol 2005;153:815-20.
[Google Scholar]
|
| 8. |
Dytoc MT, Kossintseva I, Ting PT. First case series on the use of calcipotriol-betamethasone dipropionate for morphoea. Br J Dermatol 2007;157:615-8.
[Google Scholar]
|
| 9. |
Cunningham BB, Landells ID, Langman C, Sailer DE, Paller AS. Topical calcipotriene for morphea/linear scleroderma. J Am Acad Dermatol 1998;39:211-5.
[Google Scholar]
|
| 10. |
Kerscher M, Dirschka T, Volkenandt M. Treatment of localised scleroderma by UVA1 phototherapy. Lancet 1995;346:1166.
[Google Scholar]
|
| 11. |
Gruss C, Reed JA, Altmeyer P, McNutt NS, Kerscher M. Induction of interstitial collagenase (MMP-1) by UVA-1 phototherapy in morphea fibroblasts. Lancet 1997;350:1295-6.
[Google Scholar]
|
| 12. |
Stege H, Berneburg M, Humke S, Klammer M, Grewe M, Grether-Beck S, et al. High-dose UVA1 radiation therapy for localized scleroderma. J Am Acad Dermatol 1997;36:938-44.
[Google Scholar]
|
| 13. |
Kerscher M, Volkenandt M, Gruss C, Reuther T, von Kobyletzki G, Freitag M, et al. Low-dose UVA phototherapy for treatment of localized scleroderma. J Am Acad Dermatol 1998;38:21-6.
[Google Scholar]
|
| 14. |
Gruss CJ, Von Kobyletzki G, Behrens-Williams SC, Lininger J, Reuther T, Kerscher M, et al. Effects of low dose ultraviolet A-1 phototherapy on morphea. Photodermatol Photoimmunol Photomed 2001;17:149-55.
[Google Scholar]
|
| 15. |
Sator PG, Radakovic S, Schulmeister K, Honigsmann H, Tanew A. Medium-dose is more effective than low-dose ultraviolet A1 phototherapy for localized scleroderma as shown by 20-MHz ultrasound assessment. J Am Acad Dermatol 2009;60:786-91.
[Google Scholar]
|
| 16. |
De Rie MA, Enomoto DN, de Vries HJ, Bos JD. Evaluation of medium-dose UVA1 phototherapy in localized scleroderma with the cutometer and fast Fourier transform method. Dermatology 2003;207:298-301.
[Google Scholar]
|
| 17. |
Kreuter A, Gambichler T, Avermaete A, Jansen T, Hoffmann M, Hoffmann K, et al. Combined treatment with calcipotriol ointment and low-dose ultraviolet A1 phototherapy in childhood morphea. Pediatr Dermatol 2001;18:241-5.
[Google Scholar]
|
| 18. |
Andres C, Kollmar A, Mempel M, Hein R, Ring J, Eberlein B. Successful ultraviolet A1 phototherapy in the treatment of localized scleroderma: A retrospective and prospective study. Br J Dermatol 2010;162:445-7.
[Google Scholar]
|
| 19. |
Usmani N, Murphy A, Veale D, Goulden V, Goodfield M. Photochemotherapy for localized morphoea: Effect on clinical and molecular markers. Clin Exp Dermatol 2008;33:698-704.
[Google Scholar]
|
| 20. |
Kerscher M, Meurer M, Sander C, Volkenandt M, Lehmann P, Plewig G, et al. PUVA bath photochemotherapy for localized scleroderma. Evaluation of 17 consecutive patients. Arch Dermatol 1996;132:1280-2.
[Google Scholar]
|
| 21. |
El-Mofty M, Zaher H, Bosseila M, Yousef R, Saad B. Low-dose broad-band UVA in morphea using a new method for evaluation. Photodermatol Photoimmunol Photomed 2000;16:43-9.
[Google Scholar]
|
| 22. |
El-Mofty M, Mostafa W, El-Darouty M, Bosseila M, Nada H, Yousef R, et al. Different low doses of broad-band UVA in the treatment of morphea and systemic sclerosis. Photodermatol Photoimmunol Photomed 2004;20:148-56.
[Google Scholar]
|
| 23. |
Kreuter A, Gambichler T, Breuckmann F, Rotterdam S, Freitag M, Stuecker M, et al. Pulsed high-dose corticosteroids combined with low-dose methotrexate in severe localized scleroderma. Arch Dermatol 2005;141:847-52.
[Google Scholar]
|
| 24. |
Seyger MM, van den Hoogen FH, de Boo T, de Jong EM. Low-dose methotrexate in the treatment of widespread morphea. J Am Acad Dermatol 1998;39:220-5.
[Google Scholar]
|
| 25. |
Uziel Y, Feldman BM, Krafchik BR, Yeung RS, Laxer RM. Methotrexate and corticosteroid therapy for pediatric localized scleroderma. J Pediatr 2000;136:91-5.
[Google Scholar]
|
| 26. |
Martini G, Ramanan AV, Falcini F, Girschick H, Goldsmith DP, Zulian F. Successful treatment of severe or methotrexate-resistant juvenile localized scleroderma with mycophenolate mofetil. Rheumatology 2009;48:1410-3.
[Google Scholar]
|
| 27. |
United States. Prevention Services Task Force. Guide to clinical preventive services: Report of the U.S. Preventive Services Task Force; Preface by Robert S. Lawrence. Washington: Task Force; 1989.
[Google Scholar]
|
| 28. |
Fett N, Werth VP. Update on morphea: Part II. Outcome measures and treatment. J Am Acad Dermatol 2011;64:231-42.
[Google Scholar]
|
| 29. |
Kroft EB, Creemers MC, van den Hoogen FH, Boezeman JB, de Jong EM. Effectiveness, side-effects and period of remission after treatment with methotrexate in localized scleroderma and related sclerotic skin diseases: An inception cohort study. Br J Dermatol 2009;160:1075-82.
[Google Scholar]
|
| 30. |
Weibel L, Sampaio MC, Visentin MT, Howell KJ, Woo P, Harper JI. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol 2006;155:1013-20.
[Google Scholar]
|
| 31. |
Fitch PG, Rettig P, Burnham JM, Finkel TH, Yan AC, Akin E, et al. Treatment of pediatric localized scleroderma with methotrexate. J Rheumatol 2006;33:609-14.
[Google Scholar]
|
| 32. |
Cox D, O' Regan G, Collins S, Byrne A, Irvine A, Watson R. Juvenile localised scleroderma: A retrospective review of response to systemic treatment. Ir J Med Sci 2008;177:343-6.
[Google Scholar]
|
| 33. |
Roos N, Poulalhon N, Farge D, Madelaine I, Mauviel A, Verrecchia F. In vitro evidence for a direct antifibrotic role of the immunosuppressive drug mycophenolate mofetil. J Pharmacol Exp Ther 2007;321:583-9.
[Google Scholar]
|
| 34. |
Shimizu H, Takahashi M, Takeda S, Inoue S, Fujishiro J, Hakamata Y, et al. Mycophenolate mofetil prevents transplant arteriosclerosis by direct inhibition of vascular smooth muscle cell proliferation. Transplantation 2004;77:1661-7.
[Google Scholar]
|
| 35. |
Gao R, Lu Y, Xin YP, Zhang XH, Wang J, Li YP. The effects of different immunosuppressants on chronic allograft nephropathy by affecting the transforming growth factor-beta and Smads signal pathways. Transplant Proc 2006;38:2154-7.
[Google Scholar]
|
| 36. |
Derk CT, Grace E, Shenin M, Naik M, Schulz S, Xiong W. A prospective open-label study of mycophenolate mofetil for the treatment of diffuse systemic sclerosis. Rheumatology (Oxford) 2009;48:1595-9.
[Google Scholar]
|
| 37. |
Petrova DT, Brehmer F, Schultze FC, Asif AR, Gross O, Oellerich M, et al. Differential kidney proteome profiling in a murine model of renal fibrosis under treatment with mycophenolate mofetil. Pathobiology 2011;78:162-70.
[Google Scholar]
|
| 38. |
Manzia TM, Angelico R, Toti L, Bellini MI, Sforza D, Palmieri G, et al. Long-term, maintenance MMF monotherapy improves the fibrosis progression in liver transplant recipients with recurrent hepatitis C. Transpl Int 2011;24:461-8.
[Google Scholar]
|
| 39. |
Scheel PJ Jr, Feeley N, Sozio SM. Combined prednisone and mycophenolate mofetil treatment for retroperitoneal fibrosis: A case series. Ann Intern Med 2011;154:31-6.
[Google Scholar]
|
| 40. |
Strauss RM, Bhushan M, Goodfield MJ. Good response of linear scleroderma in a child to ciclosporin. Br J Dermatol 2004;150:790-2.
[Google Scholar]
|
| 41. |
Crespo MP, Mas IB, Diaz JM, Costa AL, Nortes IB. Rapid response to cyclosporine and maintenance with methotrexate in linear scleroderma in a young girl. Pediatr Dermatol 2009;26:118-20.
[Google Scholar]
|
| 42. |
Neustadter JH, Samarin F, Carlson KR, Girardi M. Extracorporeal photochemotherapy for generalized deep morphea. Arch Dermatol 2009;145:127-30.
[Google Scholar]
|
| 43. |
Kaur S, Dhar S, Kanwar AJ. Treatment of childhood linear morphea with D-penicillamine. Pediatr Dermatol 1993;10:201-2.
[Google Scholar]
|
| 44. |
Van Bergen BH, Van Dooren-Greebe RJ, Fiselier TJ, Koopman RJ. D-penicillamine in treatment of scleroderma "en coup de sabre". Hautarzt 1997;48:42-4.
[Google Scholar]
|
| 45. |
Moinzadeh P, Krieg T, Hunzelmann N. Imatinib treatment of generalized localized scleroderma (morphea). J Am Acad Dermatol 2010;63:e102-4.
[Google Scholar]
|
| 46. |
Diab M, Coloe JR, Magro C, Bechtel MA. Treatment of recalcitrant generalized morphea with infliximab. Arch Dermatol 2010;146:601-4.
[Google Scholar]
|
Fulltext Views
23,136
PDF downloads
4,781





