Translate this page into:
Morphometric study of microvessels, epidermal characteristics and inflammation in psoriasis vulgaris with their correlations
2 Department of Dermatology, Command Hospital, Pune, India
Correspondence Address:
Biju Vasudevan
Department of Dermatology, Command Hospital, Pune-411 040
India
| How to cite this article: Boruah D, Moorchung N, Vasudevan B, Malik A, Chatterjee M. Morphometric study of microvessels, epidermal characteristics and inflammation in psoriasis vulgaris with their correlations. Indian J Dermatol Venereol Leprol 2013;79:216-223 |
Abstract
Background: Vascular proliferation, inflammation and epidermal changes are important features in the pathogenesis of psoriasis. Aims: In this study we attempted an objective evaluation of these parameters using morphometry. Methods: Inflammation, microvessels and epidermal parameters were assessed in 50 newly diagnosed cases of psoriasis vulgaris (between 01 Nov 2008 and 31 Oct 2011) by morphometry. Parameters studied were microvessel density, microvessel caliber, inflammatory cell density in dermis, ratio between inner and outer epidermal length, maximum epidermal thickness, minimum epidermal thickness and difference between maximum epidermal thickness and minimum epidermal thickness. Results: Microvessel caliber showed moderate correlation (r = 0.645) and microvessel density, weak correlation (r = 0.226) with inflammatory cell density in dermis. Both these parameters also showed mild positive correlation with "ratio between inner and outer epidermal length". All parameters except minimum epidermal thickness showed mild positive correlation with inflammatory cell density in dermis. Conclusion: All microvessels and epidermal parameters showed positive correlation with dermal inflammation; and epidermal parameters exhibited positive correlation with micro-vascular dilation. It is likely that inflammation is a key factor in the pathogenesis of psoriasis.Introduction
Psoriasis is a chronic inflammatory autoimmune disease identified by the clinicalappearance of characteristic red, raised, scaly skin lesions. It is commonly accepted that the major pathological lesions observed in psoriasis are epidermal hyperproliferation with abnormal differentiation, angiogenesis and an inflammatory infiltrate in the dermis. [1] In the past, therapies for psoriasis had focused on treating epidermal hyperplasia, which was hypothesized to occur as a result of the abnormal proliferation and differentiation of basal keratinocytes. [2] It later became evident that there was a direct role for T-cells in the pathogenesis of psoriasis. [3] Another facet to this interesting disease is the vascular proliferation which occurs in the upper dermis. Keratinocytes are thought to be a major source of pro-angiogenic cytokines (VEGF, IL-8) but the precise mechanism for angiogenesis in psoriasis is still unknown. [4]
Morphometric analysis uses special software which gives a quantitative dimension to histopathology. Histolopathology can, at the best quantify the grade of epidermal thickness as mild, moderate or severe but morphometry can give a precise quantification of the epidermal thickness. Similarly, if blood vessels are stained using the CD-34 antibody, morphometry can measure the exact diameter and cross sectional area of the blood vessel.
The quantitative correlations of microvascular and epidermal characteristics with degree of dermal inflammation in psoriasis have not been investigated. We undertook this present study to evaluate a series of psoriatic biopsies using morphometry to objectively grade the degree of angiogenesis, the amount of epidermal hyperplasia and the severity of inflammation.
Methods
A total of 50 patients of newly diagnosed and confirmed psoriasis vulgaris presenting to the dermatology department over duration of 3 years (01 Nov 2008-31 Oct 2011) were included in the study after obtaining institutional ethical clearance. Informed consent was obtained from all patients. Patients on any mode of therapy were excluded. We studied microvessel density (MVD), microvessel caliber (MVC), inflammatory cell density in dermis (INFLCD), ratio between inner and outer epidermal length (RIOEL), maximum epidermal thickness (MaxET), minimum epidermal thickness (MinET) and the difference between MaxET and MinET (DMMET).
Histopathological evaluation and immunohistochemistry for CD-34
Two pathologists independently reviewed routine hematoxylin and eosin (H and E) stained slides of all cases, and reconfirmed the original histopathological diagnoses. Representative blocks of formalin-fixed paraffin-embedded tissue were selected, 5-micrometer thick sections cut and immunohistochemical (IHC) staining performed by labeled StreptAvidin-Biotin (LSAB) technique using mouse anti-human CD-34 monoclonal antibody. Microvessels in dermis were observed by scanning sections at low power (× 100) magnification, and the area with greatest number of distinctly CD34 immunostained microvessels ("hot spot") was selected. Microvessel counting and calibre measurement were evaluated under high power magnification (× 400) in 5 fields within the hot spot (total area = 0.289 mm 2 ) [Figure - 1]a-d. H and E stained sections were used to get INFLCD and epidermal characteristics [Figure - 1]e.
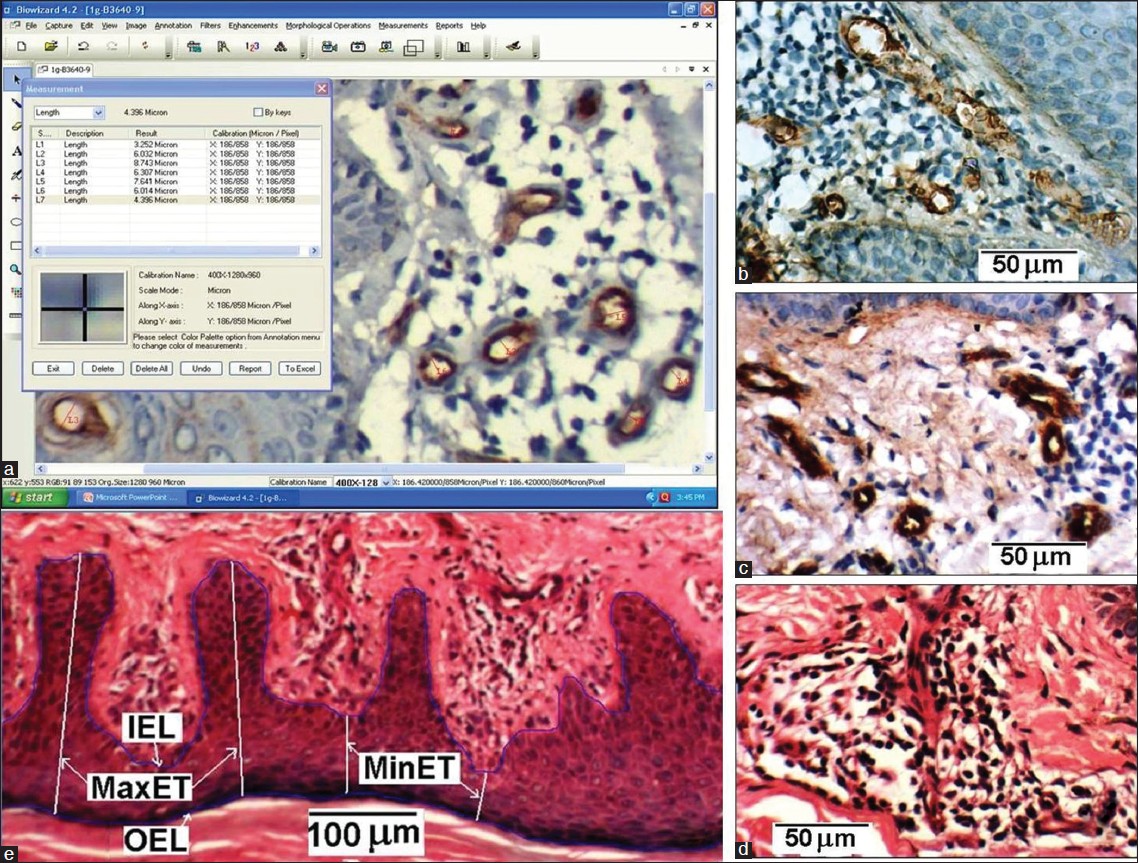 |
| Figure 1: (a) Image morphometric counting of MVD and measurement of MVC (b, c) CD34 immunostained microvessels within hot spot of dermis in two histological sections. (d) Inflammatory cells in dermis of a H and E stained section (e) Measurement scheme of epidermis parameters: IEL, OEL, MaxET and MinET with inverted epidermis for better delineation |
Vascular morphometry
Morphometric analysis was performed on CD-34 immunostained sections using a computerized digital photomicrograph system (Dewinter Optical Inc. with Digi Eye 330 digital photomicrography camera and Biowizard 4.2 Image analysis software) [Figure - 1]a. The measuring scale of image analysis software was properly calibrated with the standard scale, as per instruction given in the software manual. Images were processed using the software to get sharp microvessel boundary before performing the measurement. Images from five high power fields (400×) in the hot spot area were recorded for each sample. Microvessels in these fields were counted and the MVD was calculated. The microvessels which appeared circular and elliptical in the section were used to get mean MVC. For the elliptical case, the minor axis of the vessel was considered as its caliber. The microvessel caliber was measured using the software [Figure - 1]a.
Epidermis characteristics and degree of dermal inflammation
The H and E stained sections were used for the evaluation of epidermis characteristics. To determine epidermal thicknesses (MaxET and MinET), inner epidermal length (IEL) and outer epidermal length (OEL), three representative images of epidermis of each sample were recorded at 100 × magnification [Figure - 1]e. The distance from the crest (ridge) of the stratum basale (papillae) to the interface of stratum spinosum and stratum granulosum was considered as MinET. Similarly, the distance from the trough (trench) of the stratum basale to the interface of stratum spinosum and stratum granulosum was considered as MaxET. Five measurements of MaxET and MinET were taken for each of the three recorded 100 × fields. From the mean value of MaxET and MinET, DMMET (= MaxET - MinET) was determined, which represents the mean separation of the crest and trough of the stratum basale of the epidermis of a sample. The total length of the stratum basale in a 100 × field was taken as IEL and total length of the interface of stratum spinosum and stratum granulosum was considered as OEL. Then the ratio RIOEL (= IEL/OEL) was determined for the three recorded fields of each sample and their mean value was taken. The RIOEL is therefore a measure of the tortuousity of the basal layer.
To determine INFLCD three images of dermis region of each sample having maximum density of inflammatory cells (lymphocytes) were recorded at 400 × magnification [Figure - 1]d. The inflammatory cells in dermis were counted, the dermal area which contained those cells was measured; then (no: of inflammatory cells/dermal area) gives INFLCD. All these measurement was carried out using image analysis software.
Statistical analysis
All microvessel and epidermal parameters were statistically analysed for each sample. The mean values of these parameters with standard deviation (SD) were calculated. Data was reported as mean, SD and range of these parameters. The statistical correlations of the microvessel and epidermal parameters with INFLCD of all samples were investigated. Pearson correlation coefficient r and P value were calculated in correlation studies with regression lines. The distribution of the samples according to the evaluated parameters were also studied.
Results
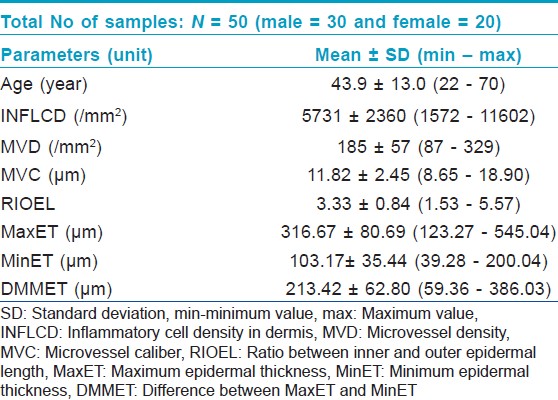
There were 30 male and 20 females among the total 50 studied cases; their mean age at the time of biopsy was 43.9 years (range: 22-70 years). The mean values of the all studied parameters with SD and range are presented in [Table - 1]. The mean INFLCD of the studied cases was 5731 mm -2 (range: 1572-11602 mm -2 ). The mean MVD was 185 mm -2 (range: 87-329 mm -2 ) and the mean MVC was 11.82 μm (range: 8.65-18.90 μm). The mean RIOEL was 3.33 (range: 1.53-5.57) and mean MaxET was 316.67 μm (range: 123.27-545.04 μm) and mean MinET was 103.17 μm (range: 39.28-200.04 μm). The mean value of DMMET was 213.42 μm (range: 59.36-386.03 μm).
Distribution of sample with studied parameters
The distribution of all samples with age, MVD, MVC, INFLCD, RIOEL, MaxET, MinET and DMMET are presented in [Figure - 2]a-h. The peak value of age distribution was found in the range: 30-40 years. The peak count in the distributions were found for: MVD in the range: 150-175 /mm 2 , MVC in the range: 10-11 μm, INFLCD in the range: 6000-7000 mm -2 , RIOEL in the range: 2.75-3.25, MaxET in the range: 325-375 μm, MinET in the range: 90-110 μm and DMMET in the range: 225-275 μm.
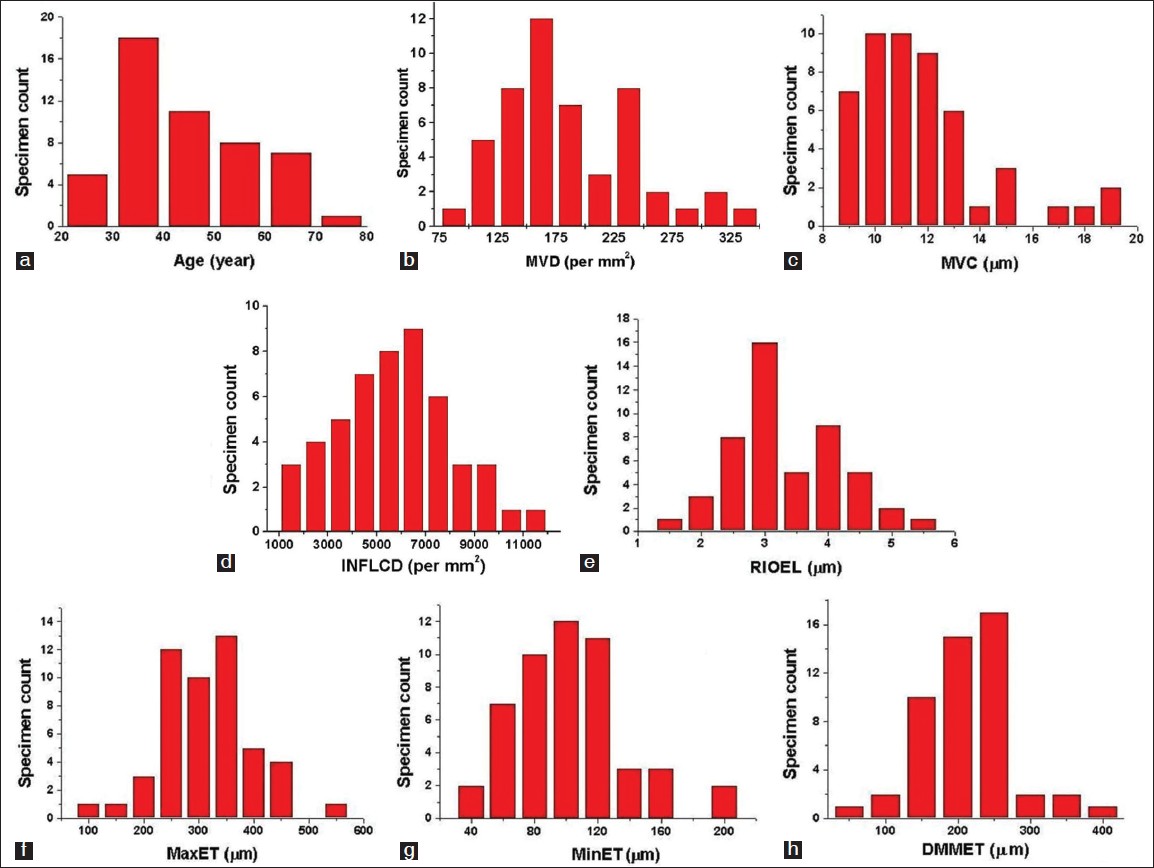 |
| Figure 2: The distribution of the studied 50 samples according to: (a) age, (b) MVD, (c) MVC, (d) INFLCD, (e) RIOEL, (f) MaxET, (g) MinET and (h) DMMET |
Linear correlations of Inflammation with vascular parameters
The MVD showed very weak positive correlation (r = 0.226, P = 0.114), whereas the MVC exhibited moderate positive correlation (0.645, P< 0.0001) with INFLCD. Scatter plots of microvessel parameters: MVD and MVC versus INFLCD for the all samples with linear regressions are presented in [Figure - 3]a,b.
 |
| Figure 3: Scatter plot of INFLCD versus (a) MVD and (b) MVC for the all samples; linear regression between these parameters and INFLCD are shown by the red lines in their respective plot |
Linear correlation of inflammation with epidermal parameters
Scatter plots of RIOEL, MaxET, MinET and DMMET versus INFLCD for the all samples with linear regressions are presented in [Figure - 4]a-d. Mild positive correlations were observed for RIOEL (r = 0.281, P = 0.048), MaxET (r = 0.327, P = 0.021) and DMMET (r = 0.358, P = 0.011) with INFLCD. But MinET (r = 0.112 P =0.437) showed no meaningful correlation with the INFLCD.
 |
| Figure 4: Scatter plot of INFLCD versus (a) RIOEL (b) MaxET, (c) MinET and (d) DMMET for the all samples; linear regression between these parameters and INFLCD are shown by the red lines in their respective plot |
Linear correlation of vascular parameters with epidermal parameters
MVD showed mild positive correlation with RIOEL (r = 0.306, P = 0.031), but all other epidermal parameters did not show significant correlation with MVD (r< 0.15, P> 0.05). The scatter plot of MVD versus RIOEL with their linear regression showed in [Figure - 5]a.
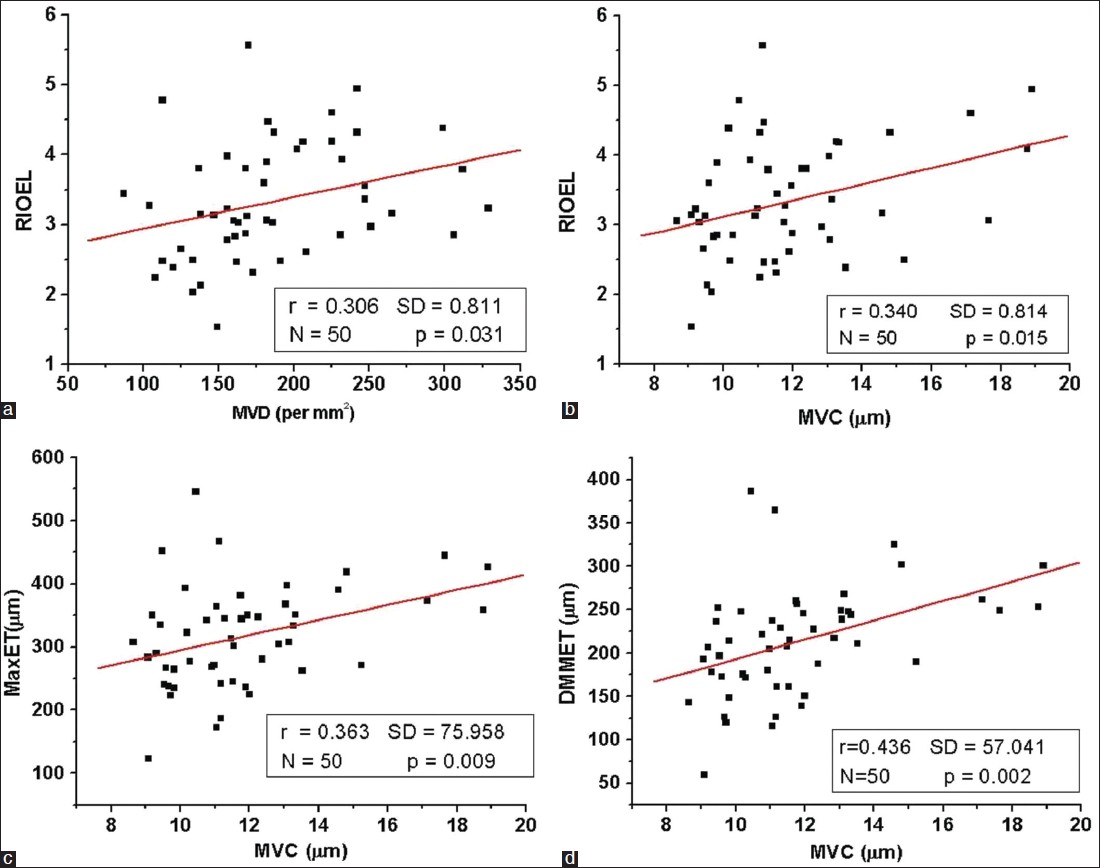 |
| Figure 5: Scatter plot of (a) MVD versus RIOEL, (b) MVC versus RIOEL, (c) MVC versus MaxET and (d) MVC versus DMMET for the all samples; linear regression between these parameters are shown by the red lines in their respective plot |
MVC showed mild positive correlation with RIOEL (r = 0.340, P = 0.015), MaxET(r = 0.363, P = 0.009), DMMET (r = 0.436, P = 0.001); but did not show any correlation with MinET (r = 0.054, P = 0.706). The scatter plot of MVC versus RIOEL, MaxET and DMMET with their linear regression is presented in [Figure - 5]b-d.
Discussion
The pathogenesis of psoriasis centers on epidermal hyperplasia, angiogenesis and inflammation. These three factors were analyzed objectively and inferences were drawn based on the data.
We found that there was a significant increase in the microvessel caliber (MVC) with the inflammatory infiltrate. The correlation between the vessel density (MVD) and inflammation showed a significantly less correlation indicating that probably rather than the proliferation of blood vessels, dilatation of preexisting blood vessels is of greater significance.
A series of experiments by Mor et al.,[5] proved that T-cells can synthesize and secrete VEGF. The role of angiogenesis as a damaging factor in retinitis due to T lymphocyte VEGF secretion has been established. [6] Our study also suggests that lymphocytes perhaps release angiogenic factors that induce capillary proliferation and dilatation. As an extension of this study, if the role of T lymphocytes in the production of VEGF in psoriasis can be established, it will further establish the correlation between capillary dilatation and inflammation.
Secondly, there was also a significant association between inflammation and epidermal thickness. This was particularly relevant to the maximum epidermal thickness and no correlation was observed with the minimal epidermal thickness. Keratinocytes can be viewed as an integral part of the skin-resident immune system; because they may act as antigen presenting cells (APCs), produce innate immune mediators, and contribute to the skin homing and local activation of immune cells. Keratinocytes respond to microbial stimuli by producing large amounts of cytokines (e.g. TNF-α, IL-1β, IL-6 and IL-18), chemotactic chemokines [e.g. IL-8 and CCL20 (CC chemokine ligand 20)] and antimicrobial peptides [e.g. HBD (Human β-Defensin)-2, HBD-3 and LL37]. [7],[8],[9] It is likely that the production of cytokines by keratinocytes is responsible for the accumulation of the inflammatory infiltrate in psoriasis.
However, the role of T-cells per se can not be ignored. It is believed that abnormal regulation of T-cells is a primary factor in the pathogenesis of psoriasis. This abnormal regulation coupled with interaction between keratinocytes and complex cytokine network is involved in the pathogenesis of the disease. [10],[11]In vitro studies show that lesional skin infiltrating CD4 + T-cells release factors that increase keratinocyte proliferation. [12] Also, an injury to defective keratinocytes could activate synthesis and release of cytokines. These cytokines are responsible for T-cell activation to a greater extent than cytokines secreted from normal epidermal cells. [13] These activated T cells then secrete cytokines and growth factors themselves which causes a further proliferation of keratinocytes and thus establish a vicious cycle of events. This cycle would explain our observation that there was a significant correlation between the degree of maximum epidermal thickness and the inflammatory infiltrate. Since the minimum epidermal thickness is not an index of epidermal proliferation, it would also explain why the degree of inflammation does not correlate with the minimal epidermal thickness.
Finally, there was also a significant correlation between the epidermal thickness and the MVC. This significant correlation extended to the maximum but not the minimum epidermal thickness. It is believed that vascular endothelial growth factor (VEGF) and interleukin-8 released from keratinocytes may contribute to the vascularization seen in psoriasis. [14] Another study showed that epidermal VEGF production is required not only for the development of dermal angiogenesis, but also as an autocrine regulator of epidermal hyperplasia. [15] In this study, we did not evaluate the VEGF or IL-8 levels; however, our findings on the correlation between epidermal thickness and MVC suggests that some angiogenic factor released by the thickened epidermis may be responsible for the capillary dilatation. It is not clear if epidermal VEGF stimulates epidermal hyperplasia directly, or through other downstream, paracrine mechanisms.
We believe that epidermal hyperplasia and inflammation are two of the factors which are responsible for the pathogenesis of psoriasis. Both epidermal hyperplasia and inflammation release cytokines which may set up a vicious cycle of events resulting in the secretion of more cytokines. This is in consonance with other studies who have suggested that psoriasis is primarily a T lymphocyte based disease. [16],[17] Several treatment modalities are now based on this concept. [18] We also suggest that epidermal VEGF is a key mediator in the pathogenesis of both angiogenesis and epidermal hyperplasia. Immunostaining for VEGF and correlating with the above parameters will confirm this hypothesis. The hypothesis that VEGF inhibitors could have a therapeutic role in psoriasis has been suggested by other authors. [19] This could also correlate with the high probability of angiogenesis being a major pathogenetic factor, as quoted by newer studies. [20]
Extensive search of literature revealed just one study which had earlier reported findings on psoriasis morphometry in detail. In this study, comparative morphometric evaluation of dermal vasculature was carried out in psoriasis and psoriasiform dermatitis. Morphometric analysis in that study revealed that length density of microvessels in psoriasis was significantly higher when compared to psoriasiform lesions. Microvessel density was also more in psoriasis than psoriasiform lesions. The study concluded that, in psoriasis there was significant vascular proliferation in response to inflammation, as indicated by tortuosity and elongation of vessels. [21]
Limitation of the study
This is a preliminary observation of various morphometric parameters in psoriasis vulgaris and their correlations. Further studies with large sample sizes will be required to substantiate all the findings and proposals for pathogenesis made in this study.
| 1. |
Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: A randomized trial. Lancet 2001;357:1842-7.
[Google Scholar]
|
| 2. |
Bayliffe AI, Brigandi RA, Wilkins HJ, Levick MP. Emerging therapeutic targets in psoriasis. Curr Opin Pharmacol 2004;4:306-10.
[Google Scholar]
|
| 3. |
Bos JD, Hulsebosch HJ, Krieg SR, Bakker PM, Cormane RH. Immunocompetent cells in psoriasis. In situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res 1983;275:181-9.
[Google Scholar]
|
| 4. |
Das RP, Jain AK, Ramesh V. Current concepts in the pathogenesis of psoriasis. Indian J Dermatol 2009;54:7-12.
[Google Scholar]
|
| 5. |
Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: Vascular endothelial growth factor is secreted by activated T Cells and induces Th1 polarization. J Immunol 2004;172:4618-23.
[Google Scholar]
|
| 6. |
Vinores SA, Chan CC, Vinores MA, Matteson DM, Chen YS, Klein DA, et al.0 Increased vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGF β) in experimental autoimmune uveoretinitis: Upregulation of VEGF without neovascularization. J Neuroimmunol 1998;89:43-50.
[Google Scholar]
|
| 7. |
Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol 2009;9:679-91.
[Google Scholar]
|
| 8. |
Gilliet M, Lande R. Antimicrobial peptides and self-DNA in autoimmune skin inflammation. Curr Opin Immunol 2008;20:401-7.
[Google Scholar]
|
| 9. |
Jansen PA, Rodijk-Olthuis D, Hollox EJ, Kamsteeg M, Tjabringa GS, de Jongh GJ, et al. Beta-defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PLoS One 2009;4:e4725.
[Google Scholar]
|
| 10. |
Ortonne JP. Recent developments in the understanding of the pathogenesis of psoriasis. Br J Dermatol 1999;140(Suppl 54) S1-7.
[Google Scholar]
|
| 11. |
Bos JD, De Rie MA. The pathogenesis of psoriasis: Immunological facts and speculations. Immunol Today 1999;20:40-6.
[Google Scholar]
|
| 12. |
Bata-Csorgo Z, Hammerberg C, Voorhees JJ, Cooper KD. Kinetics and regulation of human keratinocyte stem cell growth in short-term primary ex vivo culture. Cooperative growth factors from psoriatic lesional T lymphocytes stimulate proliferation among psoriatic uninvolved, but not normal, stem keratinocytes. J Clin Invest 1995;95:317-27.
[Google Scholar]
|
| 13. |
Chang EY, Hammerberg C, Fisher G, Baadsgaard O, Ellis CN, Voorhees JJ, et al. T cell activation is potentiated by cytokines released by lesional psoriatic, but not normal epidermis. Arch Dermatol1992;128:1479-85.
[Google Scholar]
|
| 14. |
Simonetti O, Lucarini G, Goteri G, Zizzi A, Biagini G, Lo Muzio L, et al. VEGF is likely a key factor in the link between inflammation and angiogenesis in psoriasis: Results of an immunohistochemical study. Int J Immunopathol Pharmacol 2006;19:751-60.
[Google Scholar]
|
| 15. |
Elias PM, Arbiser J, Brown BE, Rossiter H, Man MQ, Cerimele F, et al. Epidermal vascular endothelial growth factor production is required for permeability barrier homeostasis, dermal angiogenesis, and the development of epidermal hyperplasia-implications for the pathogenesis of psoriasis. Am J Pathol 2008;173:689-99.
[Google Scholar]
|
| 16. |
Christophers E. The immunopathology of psoriasis. Int Arch Allergy Immunol 1996;110:199-206.
[Google Scholar]
|
| 17. |
Schön MP, Boehncke WH. Psoriasis. N Engl J Med 2005;352:1899-912.
[Google Scholar]
|
| 18. |
Krueger GG, Callis KP. Development and use of alefacept to treat psoriasis. J Am Acad Dermatol2003;49(Suppl 2):S87-97.
[Google Scholar]
|
| 19. |
Mansouri K, Motlagh HR, Keshavarz M. Tranilast could have potential therapeutic value in the treatment of psoriasis. Med Hypotheses 2011;76:217-9.
[Google Scholar]
|
| 20. |
Heidenreich R, Röcken M, Ghoreschi K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol 2009;90:232-48.
[Google Scholar]
|
| 21. |
Gupta S, Kaur M, Gupta R, Singh S, Pant L, Singh PP. Dermal vasculature in psoriasis and psoriasiform dermatitis: A morphometric study. Indian J Dermatol 2011;56:647-9.
[Google Scholar]
|
Fulltext Views
2,866
PDF downloads
1,764





