Translate this page into:
Mucous membrane pemphigoid with antibodies against β3 subunit of laminin-332: First report from India
2 Department of Dermatology, Kurume University School of Medicine, and Kurume University Institute of Cutaneous Cell Biology, Kurume, Fukuoka, Japan
Correspondence Address:
Amrinder Jit Kanwar
Department of Dermatology, Venereology and Leprology. Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh - 160 012
India
| How to cite this article: Kanwar AJ, Vinay K, Koga H, Hashimoto T. Mucous membrane pemphigoid with antibodies against β3 subunit of laminin-332: First report from India. Indian J Dermatol Venereol Leprol 2012;78:475-479 |
Abstract
Mucous membrane pemphigoid (MMP) is a chronic, recurrent, progressive, subepidermal blistering disorder, mainly affecting the mucous membranes. Anti-laminin 332 MMP is a distinct subset of MMP with antibodies, mainly targeted against α3 or γ2 subunit. Antibodies exclusively against β3 subunit are rarely seen. An internal malignancy is frequently associated with anti-laminin 332 MMP. This disorder usually responds poorly to treatment, requiring multidisciplinary approach. Herein, we describe the first case of anti-laminin-332 MMP from India, which showed antibodies exclusively to β3 subunit.Introduction
Mucous membrane pemphigoid (MMP) is a chronic, recurrent, progressive, subepidermal blistering disorder, mainly affecting the mucous membranes. Clinically, it is characterized by erosions and permanent scarring of mucous membranes, mainly oral and conjuctival mucosae. There is variable involvement of skin with blisters and urticarial plaques, which subsequently heal with scarring. MMP is caused by autoantibodies, directed against basement membrane proteins, chiefly bullous pemphigoid antigen-2 (BP180). Other subtypes with antibodies directed against laminin-332 (also known as laminin-5 and epiligrin) and β4-integrin can also be seen. Laminin-332 is a heterotrimeric glycoprotein of 460 kDa, consisting of α3, β3 and γ2 subunits that are covalently linked by disulfide bonds. [1] In anti-laminin MMP, antibodies are mainly targeted against α3 or γ2 subunit. Antibodies to β3 subunit are rare. We describe the first Indian case of anti-laminin-332 MMP with antibodies directed exclusively against β3 subunit.
Case Report
A 60-year-old female presented with a 2-year-history of burning sensation in both eyes, progressing to limitation of eye movement and diminution of vision. She also gave a 1-year-history of recurrent, painful, oral erosions, bleeding gums and dysphagia to solid food. There was no history of cutaneous blisters and erosions or change of voice. No new medication or change in dentures prior to onset of symptoms was present. General physical examination was essentially normal. Mucocutaneous examination revealed conjuctival scarring with symblepharon formation [Figure - 1]a, erosive gingivostomatitis [Figure - 1]b and erosions over the buccal mucosa and palate. A provisional diagnosis of MMP was made, and further tests were performed to confirm the diagnosis. On laryngoscopy, the vocal cords were found to be normal and barium meal showed stricture in the mid esophagus. Light microscopy of oral mucosal biopsy showed a subepidermal cleft with a dense polymorphonuclear infiltrate [Figure - 2]. Linear deposits of IgG and C3 were found at the basement membrane zone (BMZ) on direct immunofluorescence (DIF). Indirect immunofluorescence using normal human skin and 1M NaCl-split human skin sections failed to show any circulating antibodies. Immunoblotting (IB) of recombinant protein of C-terminal domain of BP180 [2] did not reveal circulating antibodies for both IgG and IgA classes while IB of purified human laminin-332 [3] detected IgG antibodies to the 140kDa β3 subunit [Figure - 3].
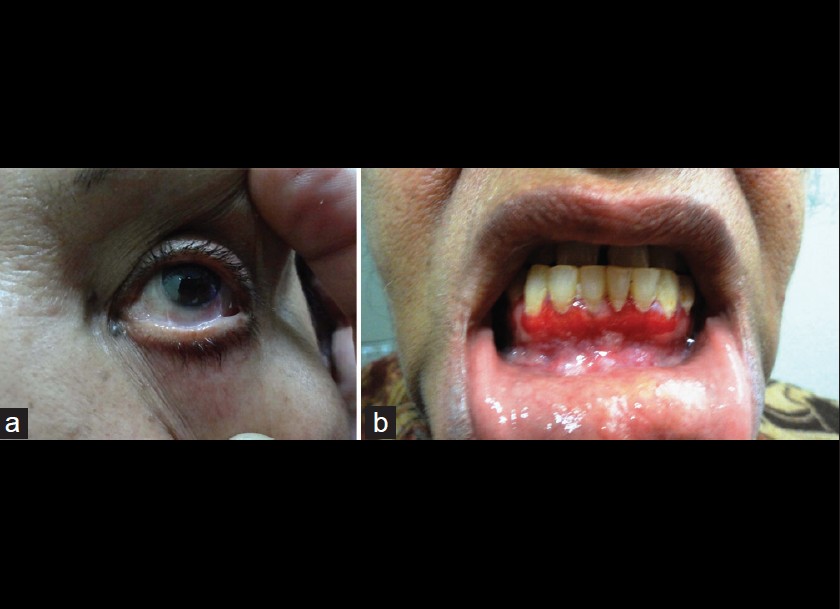 |
| Figure 1: Clinical features showing symblepharon formation (a) of the left lower conjuctiva, and erosive gingivitis (b) |
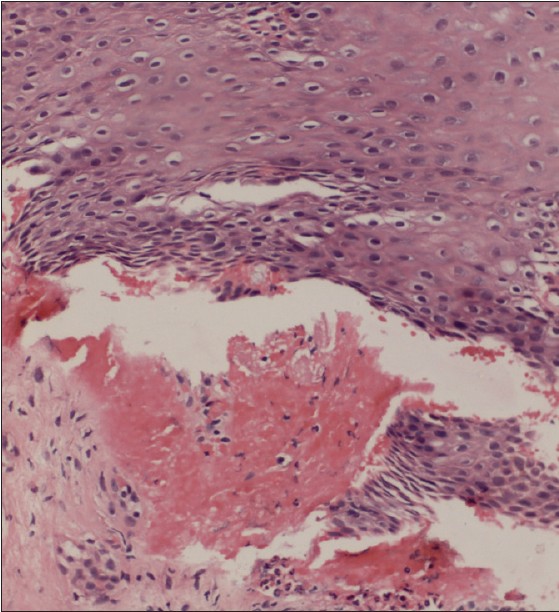 |
| Figure 2: Histopathological features of oral mucosal biopsy showing hyperplastic mucosa and subepidermal cleft containing neutrophils and fresh blood. (H and E, ×240) |
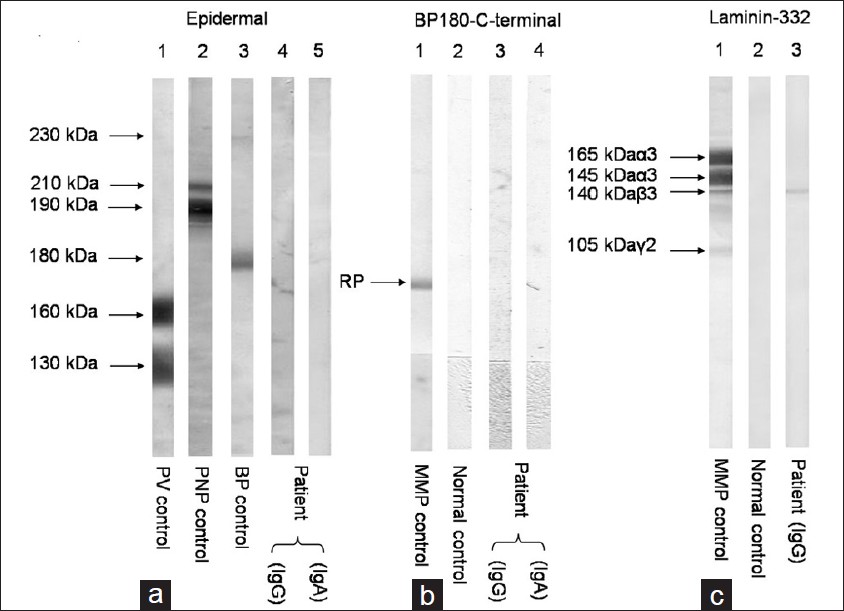 |
| Figure 3: (a) By IB of normal human epidermal extracts (Epidermal), the control pemphigus vulgaris (PV) serum reacted with the 160 kDa desmoglein 1 (Dsg1) and the 130 kDa Dsg3 (lane 1), control paraneoplastic pemphigus (PNP) serum reacted with the 210 kDa envoplakin and the 190 kDa periplakin (lane 2), and the control Bullous pemphigoid (BP) serum reacted with BP230 and BP180 (lane 3). The IgG (lane 4) and IgA (lane 5) antibodies of our case (Patient) did not react with any antigens. (b) By IB of recombinant protein of BP180 C-terminal domain (BP180 C-terminal), the control anti-BP180 type MMP serum reacted with this protein (RP) (lane 1), but normal control serum (lane 2) (normal) did not react with this protein. The IgG from our case (Patient) did not react with this protein (lane 3). (c) By IB of purified human laminin-332 (Laminin-332), the control anti-laminin-332 type MMP serum reacted with the 165 kDa and 145 kDa α 3 subunit, the 140 kDa β 3 subunit, and the 105 kDa γ 1 subunit of laminin-332 (lane 1), but normal control serum (normal) did not react with any subunits (lane 2). The IgG antibodies of our case (Patient) reacted exclusively with the 140 kDa β 3 subunit (lane 3) |
The patient was started on oral prednisolone 1 mg/ kg/ day and dapsone 100 mg. Esophageal strictures were treated with an endoscopic dilatation. Her age specific malignancy work up did not yield any significant results. Within 1 month, no new mucosal erosions developed and existing erosions started to heal. The conjuctival congestion and ocular movement also improved, but symblepharon persisted. Prednisolone was subsequently tapered off within 8 months, and dapsone was continued. The patient is completely symptom-free with no signs of disease activity on 1 year of her follow-up.
Discussion
MMP is a rare subepidermal blistering disorder, commonly affecting females in their 5 th or 6 th decade. MMP clinically has 4 distinct clinical phenotypes, associated with one of several different anti-BMZ autoantibodies. In pure ocular type, patients rarely have circulating IgG anti-BP180 antibodies, but produce IgG antibodies against the β4 integrin subunit. [4] The mucocutaneous variant shows both cutaneous and mucosal lesions and demonstrates serologic reactivity mainly against BP180. [5] The oral pemphigoid variant shows mainly oral involvement, few lesions on the skin or other mucosae may be present. Another major group shows blistering at multiple mucosal surfaces, without any cutaneous involvement. [6] Anti-laminin-332 MMP is an immunologically distinct subset affecting skin and mucous membranes. This type is associated with an increased relative risk of solid cancer.[7]
Recently, Terra et al., proposed a modified diagnostic criterion for anti-laminin-332 MMP, on clinical, histopathological and immunological basis. [8] On histopathology, MMP shows subepidermal bullae with lymphohistiocytic and eosinophilic infiltrate. Deposition of IgG in an n-serrated pattern on DIF was reported to be characteristic of anti-laminin-332 MMP. [8] Our case also showed linear deposition of immune reactants at the BMZ. Because laminin-332 is present deep in the lamina lucida localized to anchoring filaments, circulating antibodies react with dermal side of salt split human skin. By IB using purified laminin-332, antibodies directed against the subunits of laminin-332 can be determined. Commonly, antibodies are found to react with the α3 and γ2 subunit. [9] Rarely, cases of antibodies directed solely against β3 subunit have also been reported. [10],[11],[12],[13] Because of the limited literature on this entity, no clear clinical differences are seen among the cases with reactivity with different subunits. Although previous cases of anti-β3-laminin-332 MMP had both cutaneous and mucosal involvement, our case showed only mucosal lesions. Three of the previous cases of anti-β3-laminin-332 MMP had an associated neoplasm [10],[12],[13] while there was no evidence of malignancy in our patient on screening. All of the previously reported cases showed subepidermal cleft on histopathological examination with linear immune deposits at BMZ on DIF, which is consistent with our findings. Although IIF was negative in our patient, previous cases showed binding of immune deposits to the dermal side of the salt split skin. The clinical and pathological features of the reported cases of anti-β3-laminin-332 MMP has been summarized in [Table - 1].
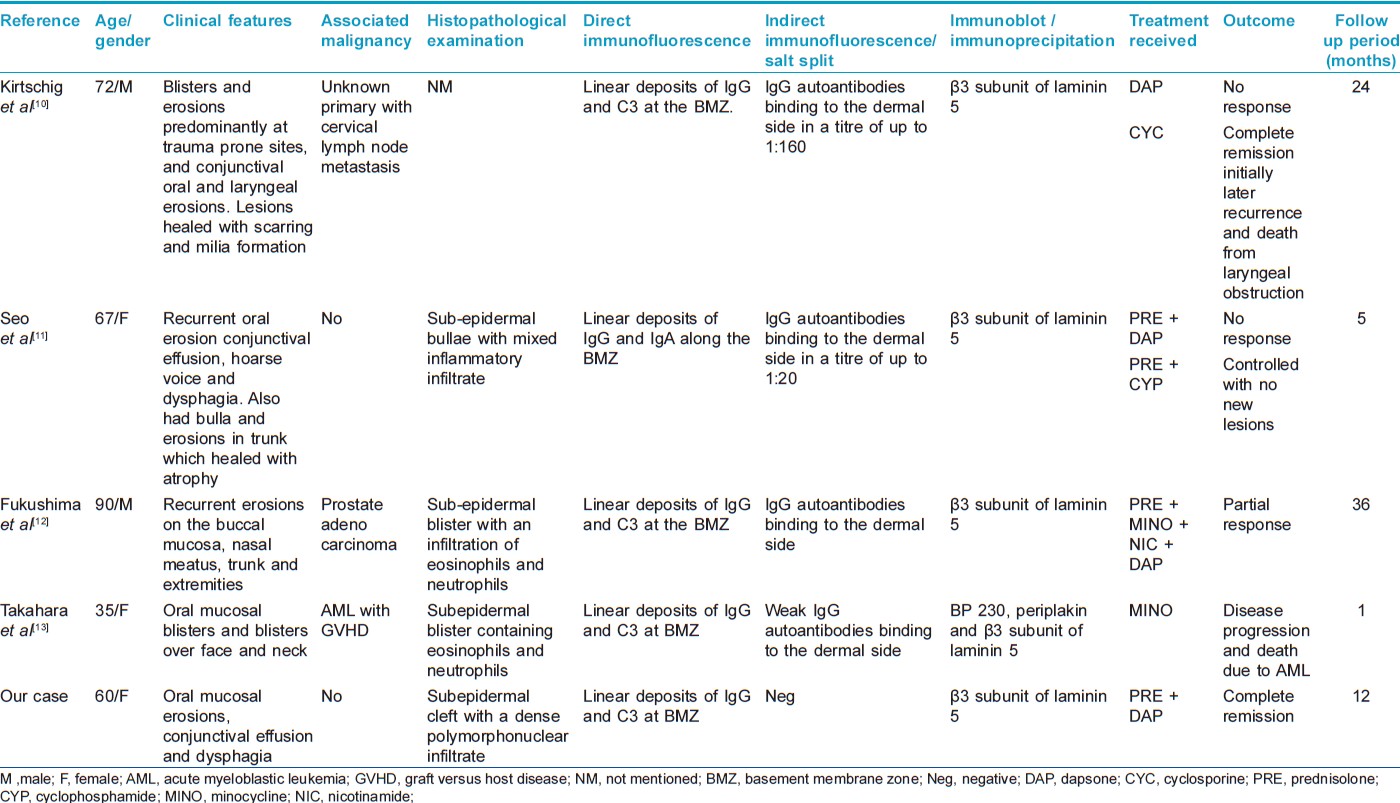
The outcome of MMP is poor compared to other subepidermal bullous disorders. Various treatment modalities, including corticosteroids, azathioprine, cyclophosphamide, doxycycline and dapsone have been used to treat MMP. Rituximab has also been tried in a few cases. [14] Previous cases of anti-β3-laminin-332 MMP have been treated with oral corticosteroids, cyclosporine, cyclophosphamide, dapsone and nicotinamide with variable response [Table - 1]. Our patient had complete clinical improvement with corticosteroids and dapsone.
In conclusion, we describe the first Indian case of anti-laminin-332 MMP. Significant morbidity associated with limited response to treatment makes MMP a challenging disease for the treating physician. Multidisciplinary approach involving the dermatologist, ophthalmologist, oral health specialist and otolaryngologist is required in caring for such patients.
| 1. |
Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, et al. A new nomenclature for the laminins. Matrix Biol 1994;14:209-11.
[Google Scholar]
|
| 2. |
Nie Z, Hashimoto T . IgA antibodies of cicatricial pemphigoid sera specifically react with C-terminus of BP180. J Invest Dermatol 1999;112:254-5.
[Google Scholar]
|
| 3. |
Hisamatsu Y, Nishiyama T, Amano S, Matsui C, Ghohestani R, Hashimoto T . Usefulness of immunoblotting using purified laminin 5 in the diagnosis of anti-laminin 5 cicatricial pemphigoid. J Dermatol Sci 2003;33:113-9.
[Google Scholar]
|
| 4. |
Tyagi S, Bhol K, Natarajan K, Livir-Rallatos C, Foster CS, Ahmed AR . Ocular cicatricial pemphigoid antigen: Partial sequence and biochemical characterization. Proc Natl Acad Sci U S A 1996;93:14714-9.
[Google Scholar]
|
| 5. |
Oyama N, Setterfield JF, Powell AM, Sakuma-Oyama Y, Albert S, Bhogal BS, et al. Bullous pemphigoid antigen II (BP180) and its soluble extracellular domains are major autoantigens in mucous membrane pemphigoid: The pathogenic relevance to HLA class II alleles and disease severity. Br J Dermatol 2006;154:90-8.
[Google Scholar]
|
| 6. |
Bruch-Gerharz D, Hertl M, Ruzicka T . Mucous membrane pemphigoid: Clinical aspects, immunopathological features and therapy. Eur J Dermatol 2007;17:191-200.
[Google Scholar]
|
| 7. |
Egan CA, Lazarova Z, Darling TN, Yee C, Cote T, Yancey KB . Anti-epiligrin cicatricial pemphigoid and relative risk for cancer. Lancet 2001;357:1850-1.
[Google Scholar]
|
| 8. |
Terra JB, Pas HH, Hertl M, Dikkers FG, Kamminga N, Jonkman MF . Immunofluorescence serration pattern analysis as a diagnostic criterion in antilaminin-332 mucous membrane pemphigoid: Immunopathological findings and clinical experience in 10 Dutch patients. Br J Dermatol 2011;165:815-22.
[Google Scholar]
|
| 9. |
Lazarova Z, Hsu R, Yee C, Yancey KB . Antiepiligrin cicatricial pemphigoid represents an autoimmune response to subunits present in laminin 5 (alpha3beta3gamma2). Br J Dermatol 1998;139:791-7.
[Google Scholar]
|
| 10. |
Kirtschig G, Caux F, McMillan JR, Bedane C, Aberdam D, Ortonne JP, et al. Acquired junctional epidermolysis bullosa associated with IgG autoantibodies to the beta subunit of laminin-5. Br J Dermatol 1998;138:125-30.
[Google Scholar]
|
| 11. |
Seo SH, Kye YC, Kim SN, Kim SC . Antiepiligrin cicatricial pemphigoid with autoantibodies to the beta subunit of laminin 5 and associated with severe laryngeal involvement necessitating tracheostomy. Dermatology 2001; 202:63-6.
[Google Scholar]
|
| 12. |
Fukushima S, Egawa K, Nishi H, Wakasugi S, Ishii N, Hashimoto T, et al. Two cases of anti-epiligrin cicatricial pemphigoid with and without associated malignancy. Acta Derm Venereol 2008;88:484-7.
[Google Scholar]
|
| 13. |
Takahara M, Tsuji G, Ishii N, Dainichi T, Hashimoto T, Kohno K, et al. Mucous membrane pemphigoid with antibodies to the beta(3) subunit of Laminin 332 in a patient with acute myeloblastic leukemia and graft-versus-host disease. Dermatology 2009;219:361-4.
[Google Scholar]
|
| 14. |
Kasperkiewicz M, Shimanovich I, Ludwig RJ, Rose C, Zillikens D, Schmidt E . Rituximab for treatment-refractory pemphigus and pemphigoid: A case series of 17 patients. J Am Acad Dermatol 2011;65:552-8.
[Google Scholar]
|
Fulltext Views
2,975
PDF downloads
1,506





