Translate this page into:
Multifocal cutaneous melanoacanthoma with ulceration: A case report with review of literature
2 Department of Pathology, Maulana Azad Medical College and Lok Nayak Hospital, New Delhi, India
Correspondence Address:
Shalu Jain
Department of Dermatology, Maulana Azad Medical College and Lok Nayak Hospital, New Delhi - 110002
India
| How to cite this article: Jain S, Barman KD, Garg VK, Sharma S, Dewan S, Mahajan N. Multifocal cutaneous melanoacanthoma with ulceration: A case report with review of literature. Indian J Dermatol Venereol Leprol 2011;77:699-702 |
Abstract
We report a case of a 58-year-old female patient who presented with multiple, asymptomatic, slowly-growing, raised pigmented lesions all over her body for the past 10 years with ulceration in one of the lesions on the trunk for the past five months. Histopathology of the lesion revealed features consistent with melanoacanthoma. Here, we report the first case of cutaneous melanoacanthoma presenting with an ulcerated plaque and the third case of cutaneous melanoacanthoma with multiple lesions. To the best of our knowledge, ulceration has not yet been reported as a feature of cutaneous melanoacanthoma in the medical literature.Introduction
Melanoacanthoma is a rare, benign, mixed tumor which clinically presents as an asymptomatic slowly-growing, raised, deeply pigmented, verrucous-surfaced, firm, round to oval plaque, papule or nodular lesions, mostly seen on the head, particularly the lips, and the trunk. [1],[2],[3],[4],[5],[6] Cutaneous melanoacanthoma most often presents as a solitary lesion but occasionally multiple lesions may also be seen. [7],[8] To the best of our knowledge, this paper describes the first case of cutaneous melanoacanthoma with ulceration which has not been described before in English language-based medical literature. We also review the literature on cutaneous melanoacanthoma with special emphasis on the multifocal lesions.
Case Report
A 58-year-old female presented to our dermatology outpatient department with complaints of multiple, asymptomatic, slow-growing, raised, pigmented lesions on her neck, chest, abdomen, back, limbs and buttocks for the past 10 years. The lesions first appeared on the trunk as small, flat and dark-colored lesions. Gradually, over a period of several months she developed similar multiple new lesions on the abdomen followed by lesions on the chest, neck, buttocks and limbs. The lesions progressively became raised, verrucous and deeply pigmented and continued to enlarge gradually until her presentation. She did not report any discomfort or itching associated with the lesions. She noticed that one of the lesions on her trunk got ulcerated five months back discharging thick yellow-colored pus leading to difficulty in lying down. She did not give any of history of trauma prior to the onset of ulceration. There was no history of bleeding, pain, induration or sudden enlargement of that lesion. There was no history of weight loss, loss of appetite or any other systemic symptoms. She had taken multiple courses of antibiotics without much relief except for slight decrease in pus discharge from the ulcerated lesion. Past and family history was not significant.
General physical examination was normal. Cutaneous examination revealed multiple, well-circumcised, hyperpigmented, firm, irregular-shaped verrucous plaques measuring 3 × 3 cm to 12 × 10 cm [Figure - 1].
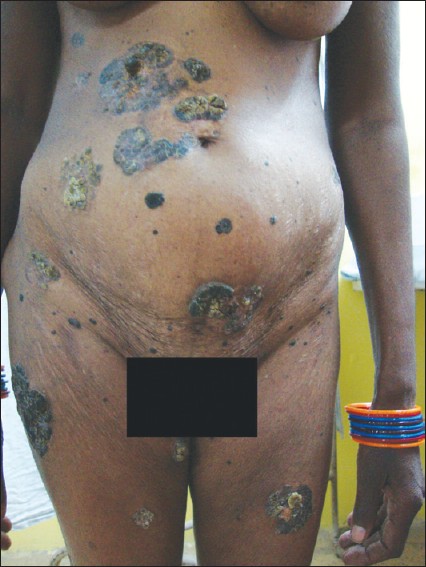 |
| Figure 1: Multiple, well-circumcised, hyperpigmented, irregular-shaped, verrucous plaques |
Lesions were located on the chest, abdomen, especially periumbilical region, trunk, arms, inner thighs and buttocks. The surface of some of the plaques, particularly on the buttocks, right shoulder and periumbilical region showed darkly pigmented, horn-like, firm, cutaneous nodules [Figure - 2]. A single, sharply demarcated, ulcerated plaque measuring 4 × 3 cm in diameter was present on the mid-back, the base of which showed pouting granulation tissue covered with dirty yellowish slough [Figure - 3]. The skin surrounding the ulcer was normal. There was no local tenderness, easy friability or bleeding on manipulation. There were also multiple, widely scattered, slightly elevated, flat-topped, dark brown-colored plaques with plugged follicular orifices on the face, neck, chest, abdomen and back measuring 0.5-2 cm giving a stuck-on appearance. Mucous membranes were spared and there was no significant lymphadenopathy. Systemic examination was within normal limits. With these clinical findings, melanoacanthoma, cutaneous melanoma and chromoblastomycosis were considered for differential diagnosis.
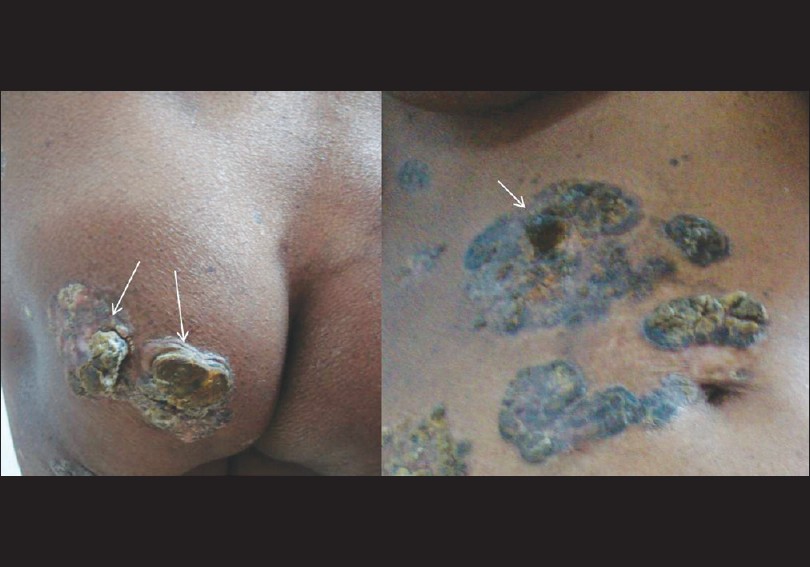 |
| Figure 2: Plaques on the left buttock and periumbilical region showing darkly pigmented, horn-like, firm cutaneous nodules (white arrows) |
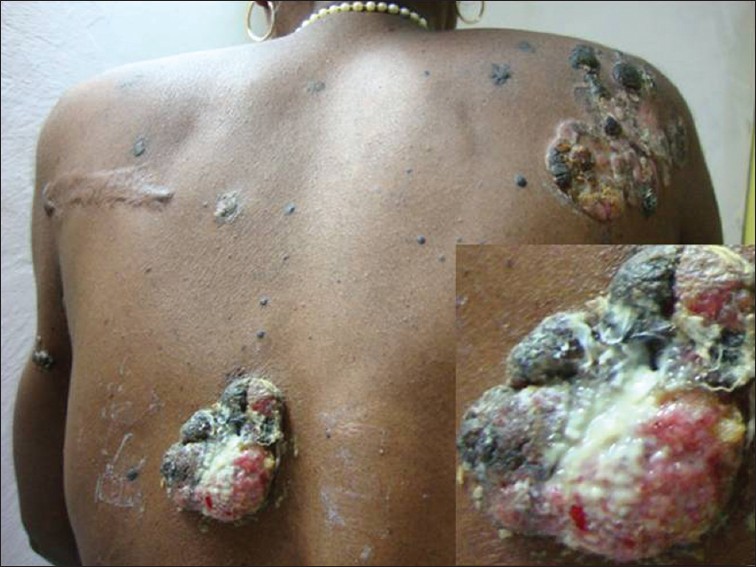 |
| Figure 3: Mid-trunk showing an ulcerated plaque of melanoacanthoma, the base of which shows pouting granulation tissue covered with dirty yellowish slough (inset focusing on ulcerated plaque). Also notice multiple flat-topped brownish-black plaques on the trunk with stuck-on appearance |
Investigations including complete blood count, fasting blood sugar level, liver function test, kidney function test and serum electrolytes were within normal limits. Venereal Disease Research Laboratory (VDRL) and human immunodeficiency virus enzyme-linked immunosorbent assay were non-reactive. A skin biopsy specimen was taken from the plaque on the abdomen for histopathology and fungal culture.
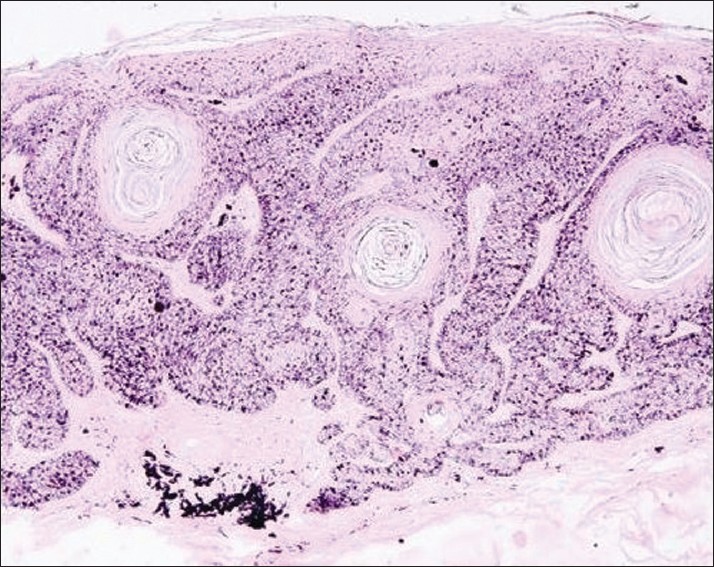
Histopathology revealed hyperkeratosis, acanthosis with tumor lobules composed of keratinocytes along with scattered large dendritic melanocytes laden with abundant melanin granules spread throughout the epidermis. Multiple horn pearls were also seen. Dermis showed focal pigment deposits in the papillary dermis [Figure - 4]. No cytologic atypia was noticed. Special stain for the melanocytes was done. The dendritic melanocytes were demonstrated by the Masson-Fontana silver impregnation stain and were seen at all the levels of the epidermis [Figure - 5]. These findings were consistent with the diagnosis of melanoacanthoma. There was no evidence of fungal cells (muriform or sclerotic cells) in the tissue section. The fungal culture was also found to be negative. Lack of invasion of surrounding tissue, mitotic activity, marked cytologic atypia and cellular variability on histopathology rules out the possibility of cutaneous malignant melanoma.
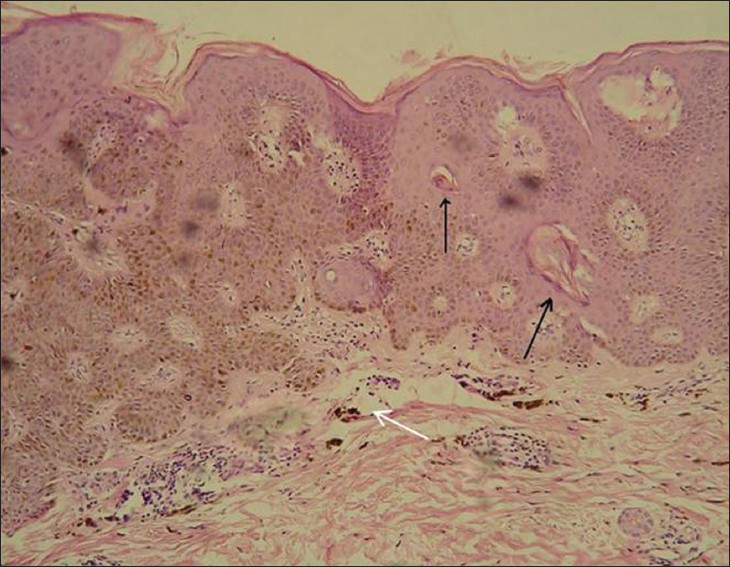 |
| Figure 4: Histopathology shows hyperkeratosis, acanthosis with scattered dendritic melanocytes in tumor lobule involving the entire epidermis. Multiple horn pearls (black arrows) are also seen along with focal pigment deposit in the papillary dermis (white arrow) (H and E, ×400) |
 |
| Figure 5: Masson-Fontana silver impregnation stain section showing dendritic melanocytes scattered throughout the epidermis (×100) |
After confirmation of diagnosis on histopathology, we started the patient on topical 5-flourouracil 5% cream to be applied twice daily over the smaller lesions, but there were no significant improvement after four weeks and patient discontinued further treatment due to burning sensation at the site of application. After that we planned for surgical excision and skin grafting for ulcerated plaque, but she refused the surgery.
Discussion
Melanoacanthoma is a rare, pigmented, benign, mixed neoplasm, composed of pigment-forming dendritic melanocytes and proliferating epidermal keratinocytes. Mishima and Pinkus [9] introduced the term melanoacanthoma to describe this benign condition although prior to this designation, Bloch [10] described a similar case in 1927 as "non-naevoid melanoepithelioma Type 1." Melanoepithelioma Type 2 is the term used to describe ordinary pigmented seborrhoeic keratosis.
Cutaneous melanoacanthoma usually occurs in elderly individuals, with the average age of involvement being 55-65 years, with no sex predilection. Whites are more commonly affected than their black counterparts. [11],[12] Although most considered cutaneous melanoacanthoma to be a benign mixed tumor of melanocytes and keratinocytes, some authors have suggested that it may represent a reactive phenomenon induced by local irritation or trauma, especially on the lips. [5] Cutaneous melanoacanthoma presents clinically as an asymptomatic, slowly-growing, well-demarcated, deeply pigmented, hyperkeratotic, dull-looking, verrucous-surfaced, round or oval papule, plaque or nodular lesions. Occasionally, it can also present as a cutaneous horn. [13] Mostly lesions are sessile but occasionally may be pedunculated, particularly in the major flexures. [7] The lesions vary in size with a diameter ranging from a few millimeters to several centimeters and are most frequently found on the head, particularly the lips, and the trunk. [1],[2],[3],[4],[5],[6] In our case the lesions of cutaneous melanoacanthoma were large, up to 12 ×10 cm in size and were located on unusual sites like the buttocks, and the proximal part of the limbs. Cases reported recently in the literature have described cutaneous melanoacanthoma involving the eyelid, [13] oral mucosa, [14],[15] and genital and perianal region. [7] Two cases of involvement of the penile shaft by cutaneous melanoacanthoma have also been described. [7],[11] Cheng et al, described a case of melanoacanthoma of the external auditory canal. [16] Kihiczak et al, reported a patient with giant cutaneous melanoacanthoma that presented as a cutaneous horn extending from the left upper eyelid downward to below the lower eyelid obstructing his vision. [13]
Cutaneous melanoacanthoma usually presents as a solitary lesion, though two cases with multiple lesions of cutaneous melanoacanthomas have been reported in the literature. [7],[8] Shenoy et al, reported a patient with multifocal cutaneous melanoacanthoma involving the lower abdominal wall, root and shaft of penis, left inner thigh and perianal skin. [7] Spott et al, reported another case of multiple cutaneous melanoacanthoma in a 40-year-old man who had multiple, small, discrete as well as confluent papules of melanoacanthoma limited to his left upper eyelid. [8] Our patient also had multiple lesions of melanoacanthoma distributed widely over the chest, abdomen, trunk, proximal limbs and the buttocks.
Histopathologically melanoacanthoma is characterized by numerous well-defined islands of small basaloid cells intermingled with large dendritic melanocytes spread throughout the epidermis. The epidermis is usually acanthotic, hyperkeratotic and small horn pearls may also be seen. The melanocytes are large, richly dendritic and contain abundant melanin granules and are scattered throughout the tumor lobules of a melanoacanthoma rather than restricted to the basal layer of the epidermis. [17] Two histologic variants of melanoacanthomas have been described: a diffuse type in which melanocytes are unevenly scattered throughout the lesion and a clonal type in which melanocytes and keratinocytes are clustered in small nests. [12] The transfer of melanin from these highly dendritic melanocytes to mature keratinocytes is often partially blocked, though in some cases nearly all the melanin is retained in the melanocytes. [17],[18]
It is also interesting to mention that in our patient, one of the plaques of melanoacanthoma was ulcerated. To the best of our knowledge, ulceration in melanoacanthoma has not been reported previously in the literature. We hypothesized that the large size of the lesion leading to compromised blood flow, trauma, friction and pressure while lying down led to the development of ulceration in our patient.
Literature on melanoacanthoma usually refers to its origin, clinical features and histopathological characteristics with paucity of literature on the treatment options. There is no effective medical treatment available for cutaneous melanoacanthoma. As melanoacanthoma is a benign lesion, local surgical excision is generally curative. Cryotherapy and laser ablation are other treatment modalities though they lack the benefit of histologic confirmation of removed lesion. Curettage has also been used. In our case, the patient had multiple lesions so the above mentioned treatment modalities were not practically possible. We tried 5% topical 5-flourouracil cream on the lesions but it was discontinued later due to lack of significant improvement and local side-effects.
| 1. |
Simon P, Requena L, Sanchez Yus E. How rare is melanoacanthoma? Arch Dermatol 1991;127:583-4.
[Google Scholar]
|
| 2. |
Reaves CE. Melanoacanthoma: A case report and review of the literature. Bull Assoc Milit Dermatol 1972;22:55-8.
[Google Scholar]
|
| 3. |
Matsuoka LY, Glasser S, Barsky S. Melanoacanthoma of the lip. Arch Dermatol 1979;115:1116-7.
[Google Scholar]
|
| 4. |
Lambert WC, Lambert MW, Mesa ML, Schnieder LC, Fischman GJ, Abbey AH, et al. Melanoacanthoma and related disorders. Simulants of acral-lentiginous (P-P-S-M) melanoma. Int J Dermatol 1987;26:508-10.
[Google Scholar]
|
| 5. |
Matsuoka LY, Barsky S, Glasser S. Melanoacanthoma of the lip. Arch Dermatol 1982;118:290.
[Google Scholar]
|
| 6. |
Sexton FM, Maize JC. Melanotic macules and melanoacanthomas of the lip. A comparative study with census of the basal melanocytes population. Am J Dermatopathol 1987;9:438-44.
[Google Scholar]
|
| 7. |
Shenoy MM, Teerthanath S, Bhagavan KR. Genital and perianal melanoacanthomas. Indian J Dermatol 2007;52:109-10.
[Google Scholar]
|
| 8. |
Spott DA, Heaton CL, Wood MG. Melanoacanthoma of the eyelid. Arch Dermatol 1972;105:898-9.
[Google Scholar]
|
| 9. |
Mishima Y, Pinkus H. Benign mixed tumor of melanocytes and malpighian cells. Melanoacanthoma: Its relationship to Bloch's benign non-nevoid melanoepithelioma. Arch Dermatol 1960;81:539-50.
[Google Scholar]
|
| 10. |
Bloch B. Ueber benigne, nicht naevoide Melanoepitheliome der Haut nebst Bemerkungen uber das Wesen und die Genese der Dendritenzellen. Arch Dermatol Syph (Berlin) 1927;153:20-40.
[Google Scholar]
|
| 11. |
Vion B, Merot Y. Melanoacanthoma of the penis shaft. Report of a case. Dermatologica 1989;179:87-9.
[Google Scholar]
|
| 12. |
Prince C, Mehregan AH, Hashimoto K, Plotnick H. Large melanoacanthomas: A report of five cases. J Cutan Pathol 1984;11:309-17.
[Google Scholar]
|
| 13. |
Kihiczak GG, Centurion SA, Schwartz RA, Lambert WC. Giant cutaneous melanoacanthoma. Int J Dermatol 2004;43:936-7.
[Google Scholar]
|
| 14. |
Fornatora ML, Reich RF, Haber S, Solomon F, Freedman PD. Oral melanoacanthoma: A report of 10 cases, review of the literature, and immunohistochemical analysis for HMB-45 reactivity. Am J Dermatopathol 2003;25:12-5.
[Google Scholar]
|
| 15. |
Flaitz CM. Oral melanoacanthoma of the attached gingiva. Am J Dent 2000;13:162.
[Google Scholar]
|
| 16. |
Cheng AG, Deubner H, Whipple ME. Melanoacanthoma of the external auditory canal: a case report and review of the literature. Am J Otolaryngol 2007;28:433-5.
[Google Scholar]
|
| 17. |
Schlappner OL, Rowden G, Phillips TM, Rahim Z. Melanoacanthoma. Ultrastructural and immunological studies. J Cutan Pathol 1978;5:127-41.
[Google Scholar]
|
| 18. |
Delacretaz J. Melano-acanthome. Dermatologica 1975;151:236-40.
[Google Scholar]
|
Fulltext Views
8,928
PDF downloads
1,927





