Translate this page into:
Multiple eruptive pilomatricomas in a young woman with glioblastoma multiforme
Corresponding author: Dr. R. Sivayogana, Department of Dermatology, Venereology and Leprosy, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India. drsivayogananew1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Harshatha S, Sivayogana R, Manuel A, Murugan S. Multiple eruptive pilomatricomas in a young woman with glioblastoma multiforme. Indian J Dermatol Venereol Leprol 2021;87:86-9.
Sir,
Pilomatricoma is a benign tumor arising from the hair follicle cortex cells. Although they usually occur as a solitary tumor, about 5% of cases can present as multiple lesions. Multiple lesions usually have familial inheritance and can have syndromic associations such as myotonic dystrophy, Curschmann-Steinert syndrome, Gardner syndrome, Rubinstein-Taybi syndrome, Turner’s syndrome, Sotos syndrome and gliomatosis cerebri.1 Herein, we present a rare association of multiple eruptive pilomatricomas in a 20-year-old woman with glioblastoma multiforme. We found only a single case report of a similar association published so far in the literature.
A 20-year-old woman presented to our dermatology department with a sudden onset of widespread, multiple asymptomatic raised skin lesions for 2 months. Initially, the lesions were pea-sized but later progressed to attain the present size as depicted in Figures 1a-c. She was diagnosed with glioblastoma multiforme of the left temporal lobe 2 years back. She underwent surgical excision and completed radiotherapy and chemotherapy with temozolomide and phenytoin for seizures. Following this, magnetic resonance imaging was performed 6 months later, which showed complete remission of the tumor. There swas no significant family history. The systemic examination was normal. Dermatological examination revealed multiple erythematous to skin-colored nodules on the face, trunk, upper and lower limbs. Lesions were non-tender, firm to hard in the consistency of size ranging from 0.5 × 0.5 cm to 5 × 3 cm. A few lesions showed bluish discoloration in the center and a few other lesions had central white hard areas indicating calcium deposition. There were no ocular, oral, or nail lesions. The patient did not exhibit any features of the abovementioned syndromes. Differential diagnoses considered were pilomatricoma, steatocystoma, bone tumors, epidermoid cyst and sebaceous carcinoma. All baseline investigations were normal. Skin biopsy from one of the representative lesions, showed a partially circumscribed lesion composed of keratin, ghost cells and intermediate cells, with areas of calcification suggestive of pilomatricoma. There was no evidence of granuloma or malignancy [Figure 2a]. Glioblastoma tissue showed multiple fragments of neural tissue with adjacent cellular lesion with moderate atypia, vascular endothelial proliferation, hemorrhage and atypical mitotic figures [Figure 2b]. She underwent excision of cosmetically disfiguring lesions. Repeat magnetic resonance imaging was done 1 year later. It revealed gliotic changes in the left posterior temporal lobe suggestive of recurrence [Figure 3]. For this, she is being carefully monitored and managed conservatively under neurosurgeons.
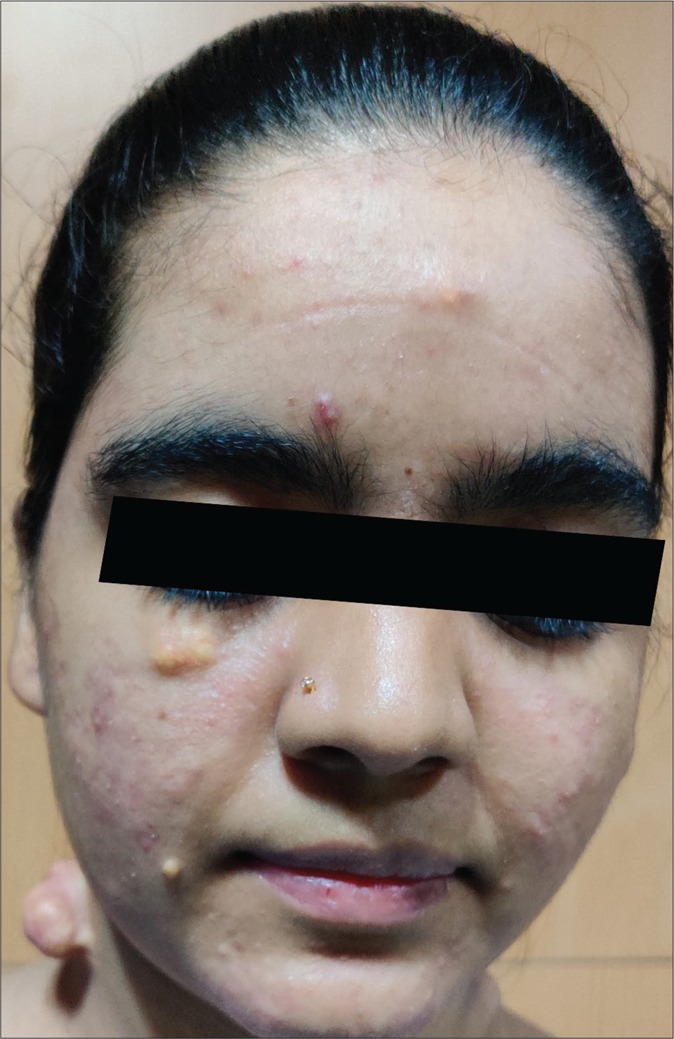
- Multiple eruptive pilomatricomas of varying size on face
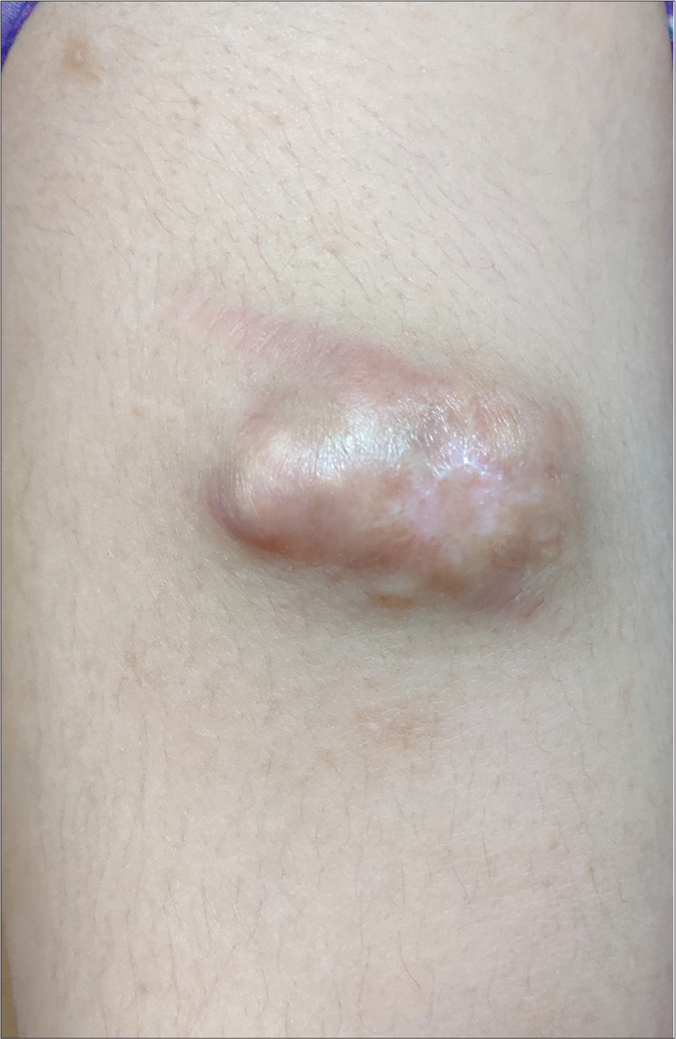
- Well-defined erythematous plaque with central areas of calcification on the right arm
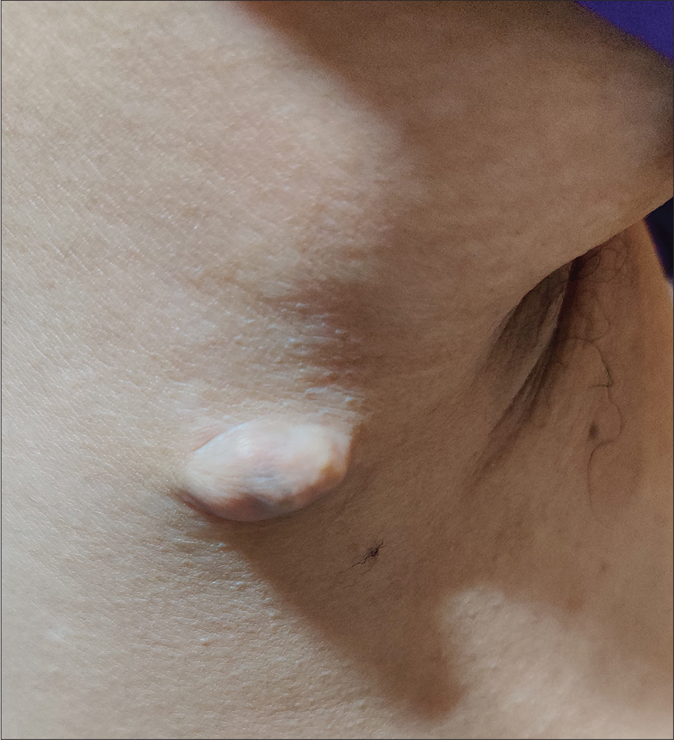
- Well-defined plaque with bluish discoloration in the center near the right axillary region
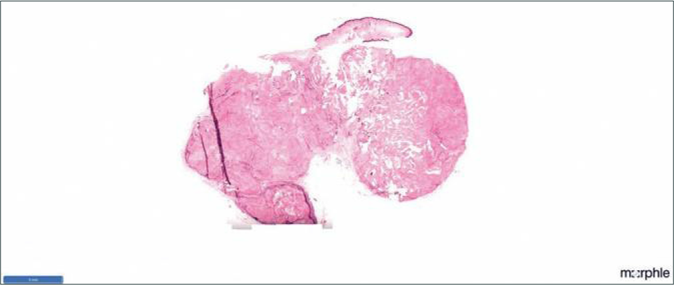
- HPE of pilomatricoma in scanner view
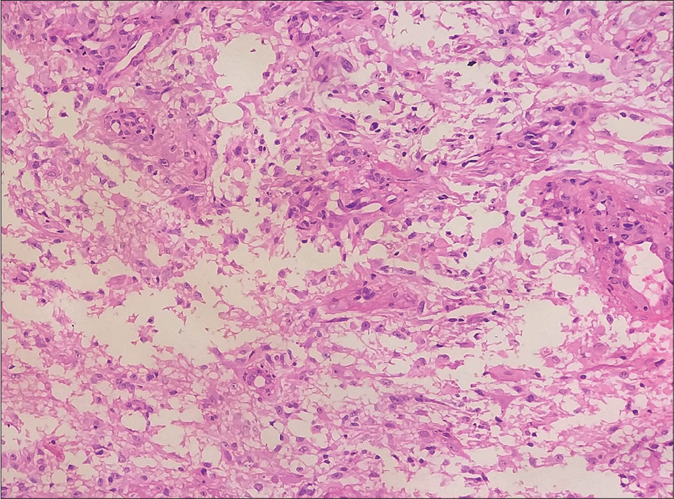
- Glioblastoma multiforme showing moderate atypia, vascular endothelial proliferation, hemorrhage and atypical mitotic figures (H and E, ×400)
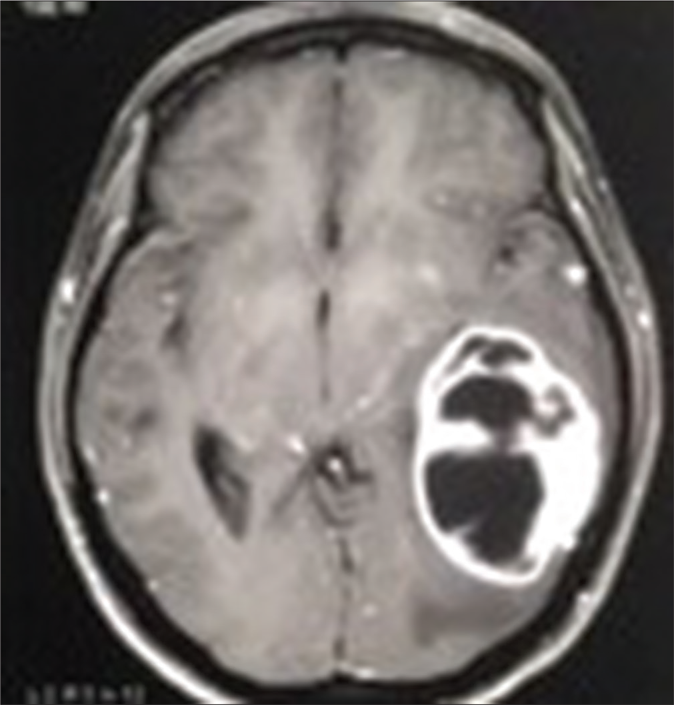
- Magnetic resonance imaging-brain – glioblastoma multiforme tumor in posterior temporal lobe before excision
Glioblastoma multiforme is a rare, rapidly growing, lethal brain tumor that belongs to the World Health Organization grade IV classification with a median survival period of only 14–15 months from the period of diagnosis.2 Pilomatricoma occurring in the setting of glioblastoma, is extremely rare as only a single case has been reported worldwide.3 Nuclear localization of the β catenin gene in basophilic cells is responsible for cellular proliferation in pilomatricoma and it is shown to be associated with a mutation in the β-catenin gene (most common being mutation in exon-3). Mutation in the β-catenin gene further causes Wnt signaling pathway activation.4 Recent exploration has also established strong upregulation of the β-catenin gene in glioblastoma multiforme. This leads to the aggressive nature of the tumor, signifying a poor prognostic sign.5 We suspected that similar β-catenin mutation was responsible for the development of pilomatricoma and recurrence of glioblastoma multiforme in our patient. Moreover, in the present case, there were no features of muscle dystrophy, muscle weakness, epidermoid cysts and osteomas, hypertrophic scars and keloid, cutis laxa and pterygium colli. By this, we have ruled out other genetic causes of tumorigenesis like myotonic dystrophy (dystrophia myotonica protein kinase gene),
Curschmann-Steinert syndrome (dystrophia myotonica protein kinase gene), Gardner syndrome (adenomatous polyposis coli gene), Rubinstein-Taybi syndrome (cAMP-response element-binding protein) and Turner’s syndrome (chromosome 45, X0), respectively.6 Since there was a proven association between pilomatricoma and glioblastoma from a previous case report,3 immunohistochemical staining for β-catenin was carried on the pilomatricoma and the brain tissue and this was strongly positive. It showed the staining of the membrane, cytoplasmic and nuclear compartments of both the lesions [Figure 4a and b]. Unfortunately, we were unable to confirm it by genetic analysis, as our patient did not consent for it. Apart from the association of pilomatricoma with glioblastoma multiforme, Wachter has reported an association between multiple pilomatricomas and gliomatosis cerebri. In this report also, the role of β-catenin mutation in tumorigenesis has been stressed.6
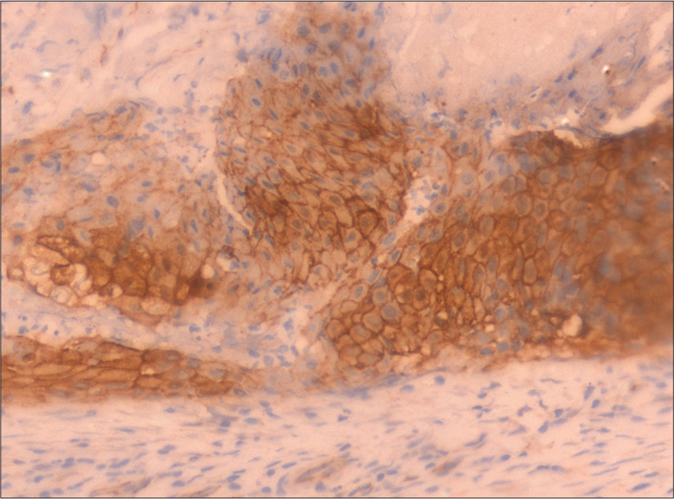
- Pilomatricoma tissue showing strong uptake of β-catenin (Immunohistochemistry, ×400)
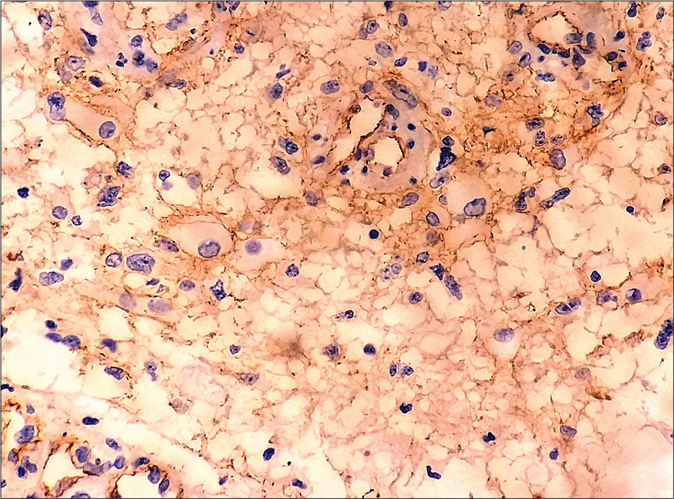
- Strong β-catenin staining of glioblastoma tissue (Immunohistochemistry, ×400)
We report this case to highlight the new rare association of glioblastoma multiforme and pilomatricoma which is not a mere coincidence, but has a strong genetic link concerning β-catenin and Wnt signaling pathway activation. Therefore, we hypothesize that the development of multiple eruptive pilomatricomas could be a cutaneous marker of recurrent and aggressive glioblastoma multiforme in the future.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal the identity but anonymity cannot be guaranteed.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Tumors of the epidermal appendages In: Elder DE, Elenitsas R, Rosenbach M, eds. Lever's Histopathology of the Skin (11th ed). Philadelphia: JB Lippincott Williams and Wilkins; 2015. p. :1054-6.
- [Google Scholar]
- Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18:3-9.
- [Google Scholar]
- Multiple eruptive pilomatricomas in a 9-year-old boy with glioblastoma. Pediatr Dermatol. 2013;30:756-8.
- [CrossRef] [PubMed] [Google Scholar]
- Beta-catenin mutation and its nuclear localization are confirmed to be frequent causes of Wnt signaling pathway activation in pilomatricomas. J Dermatol Sci. 2006;41:67-75.
- [CrossRef] [PubMed] [Google Scholar]
- ?-Catenin overexpression in malignant glioma and its role in proliferation and apoptosis in glioblastma cells. Med Oncol Northwood Lond Engl. 2011;28:608-14.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple pilomatricomas and gliomatosis cerebri-A new association? Pediatr Dermatol. 2009;26:75-8.
- [CrossRef] [PubMed] [Google Scholar]





