Translate this page into:
Nilotinib-induced psoriasis in a patient of chronic myeloid leukemia responding to methotrexate
2 Department of Internal Medicine, Division of Oncology, Dayanand Medical and Hospital, Ludhiana, India
3 Department of Pathology, Dayanand Medical and Hospital, Ludhiana, India
Correspondence Address:
Sukhjot Kaur
Department of Dermatology and Venereology, Dayanand Medical College and Hospital, Ludhiana
India
| How to cite this article: Kaur S, Arora AK, Sekhon JS, Sood N. Nilotinib-induced psoriasis in a patient of chronic myeloid leukemia responding to methotrexate. Indian J Dermatol Venereol Leprol 2015;81:216-218 |
Sir,
Epidermal growth factor receptor (EGFR) inhibitors are a group of targeted chemotherapeutic agents which are currently used in the treatment of many malignancies. [1] They cause attenuation in the EGFR signaling pathways that regulate cell differentiation, proliferation, migration, angiogenesis, and apoptosis. Tyrosine kinase inhibitors (TKI) are a class of EGFR inhibitors that act by inhibiting the autophosphorylation of tyrosine residues of several proto-oncogenes by binding to the kinase domain of different oncogenic tyrosine kinases, and therefore inhibit the activation of the intracellular signal transduction pathway in tumor cells. Thus, they lead to deregulation of key cell functions such as proliferation and differentiation. [1]
These targeted agents are associated with a wide spectrum of dermatological toxicities because they not only target tumor-related growth factors but also target growth factors in the skin and its appendages. These cutaneous adverse effects tend to be uncomfortable, disfiguring, and adversely affect the quality of life and compliance with anti-EGFR therapy. [1],[2] Although there are several reports of psoriasis developing with the first-generation tyrosine kinase inhibitor, imatinib, [2],[3] development of psoriasis in a patient on nilotinib, a second-generation tyrosine kinase inhibitor, has been described only once before. [4] Here, we present another patient with chronic myeloid leukemia who developed psoriasis while on nilotinib therapy and improved with methotrexate.
A 30-year-old man reported to the dermatology department with multiple erythematous scaly plaques over his trunk and extremities for 1 month. The lesions had gradually increased in size and number and spread over the body with mild itching. He was a known case of imatinib-resistant chronic myeloid leukemia, being treated with nilotinib 300 mg (Tasigna, AMN107), twice daily for 4 months. There was no personal or family history of similar skin lesions. He was not on any other medications and denied any other illness. Examination revealed multiple bilaterally symmetrical, well-defined erythematous plaques of varying sizes with characteristic silvery-white scales over the scalp, trunk, upper limbs, lower limbs, and hands [Figure - 1] and [Figure - 2]. Nails and joints were normal. Histopathological examination revealed hyperkeratosis, acanthosis, loss of granular layer, suprapapillary thinning, dilated papillary dermal vessels, and a perivascular chronic inflammatory infiltrate in the dermis along with a few dermal neutrophils, consistent with psoriasis vulgaris [Figure - 3]. There were no interface changes, exocytosis of lymphocytes, eosinophils, or neutrophils, and no eosinophils or plasma cells could be observed in the dermis. Treatment with topical fluticasone propionate 0.05% and topical calcitriol ointment was initiated. However, after 2 weeks of topical treatment, there was no significant improvement. Since he could not attend twice-weekly sessions for phototherapy, treatment with 15 mg of methotrexate weekly and 5 mg folic acid twice weekly was initiated. This was continued for the next 6 weeks with close monitoring, along with topical fluticasone propionate 0.05% cream. Skin lesions improved with decrease in erythema and scaling. Currently his psoriasis is well controlled with treatment. Nilotinib has been continued without any change in the dose.
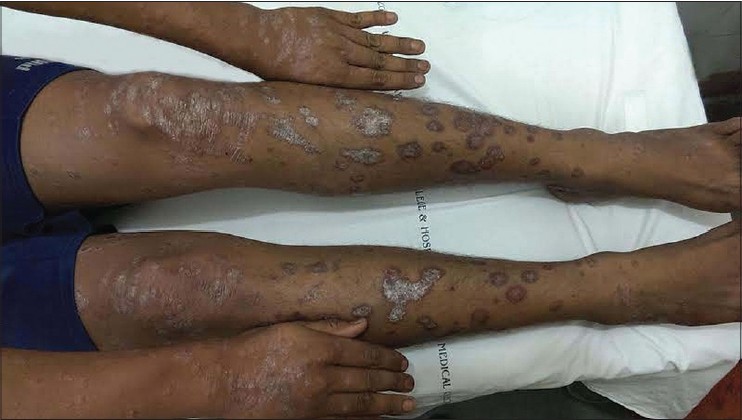 |
| Figure 1: Erythematous plaques with silvery white scales on extremities |
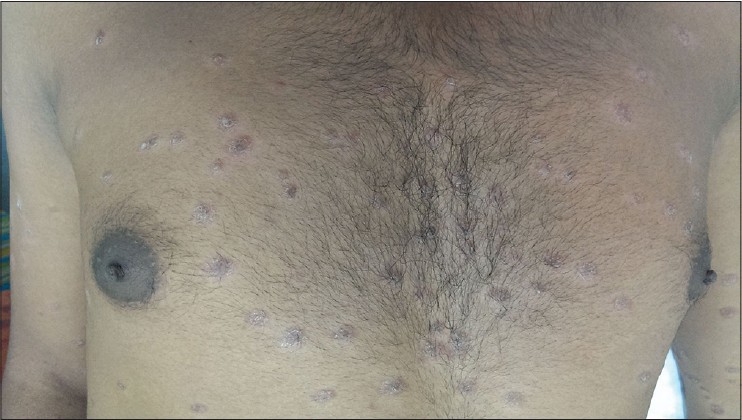 |
| Figure 2: Multiple scaly erythematous plaques on the trunk |
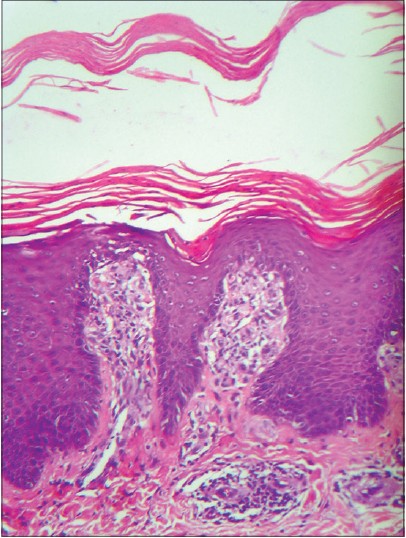 |
| Figure 3: Hyperkeratosis, acanthosis, suprapapillary thinning, and chronic perivascular inflammatory infiltrate in the dermis. (H and E, ×200) |
Nilotinib, a second-generation tyrosine kinase inhibitor, is indicated for imatinib-resistant chronic myeloid leukemia. [5] It has a higher binding affinity than imatinib for tyrosine kinases like Bcr-abl, platelet-derived growth factor receptor, and c-KIT. [6] Cutaneous side effects constitute the most common non-hematological adverse events observed with nilotinib, and most of these rashes are mild to moderate in severity. [2],[7] The incidence of rash with nilotinib varies from 22 to 62%, usually manifesting as morbilliform eruption, erythema and scaling, keratosis pilaris like lesions, and multiple milia-like lesions, associated with pruritus in 75% cases. [7],[8] Other rare adverse effects reported include development of Sweet′s syndrome, an intensified inflammatory reaction of actinic keratosis after 5-fluorouracil (5-FU) treatment, rosacea-like eruption, dry skin, and alopecia. [6],[7] The uncommon adverse effects like eczema, urticaria, hyperhidrosis, erythema nodosum, skin ulcer, petechiae, photosensitivity, ecchymosis, and swelling of face that have been reported with nilotinib are mentioned in the package insert of the drug. [2],[9]
Several reports describe aggravation or development of psoriasis with the first-generation tyrosine kinase inhibitor, imatinib, [2],[3] although paradoxical improvement of psoriasis has also been described with it. [10] Recently, Nagai et al. reported the first case of psoriasis in a patient of chronic myeloid leukemia on nilotinib therapy who responded to topical vitamin D3 analogs and corticosteroids, and nilotinib administration could be continued without dose reduction. [4] Psoriasis is a chronic relapsing inflammatory disease in which lymphocytes like Th1, Th17, and their cytokines play an important pathogenetic role. The activity of these T cells is modulated by T regulatory (Treg) cells. [11] Normally, Treg cells are able to inhibit the immunological response and maintain cutaneous immunological homeostasis, thereby preventing an autoimmune response against self-antigens. [11] In psoriasis, the suppressor activity of Treg cells is decreased, either due to a reduction in the number of these cells, a reduced ability of Treg cells to produce suppressive cytokines or "resistance" of T effector cells to their inhibition. [11]
Both imatinib and nilotinib have been demonstrated to impair the function of Treg cells in a dose-dependent manner. Chen et al. and Larmonier et al. demonstrated that the proliferation of Treg cells and their immunosuppressive effect is inhibited by imatinib at a concentration achieved clinically (2.5-5 μM). [12],[13] Similarly, nilotinib has been shown to inhibit phytohemagglutin in induced CD8+ T cell proliferation in therapeutic concentrations (0.5-4 μM) [14] and also to suppress lymphocyte-specific protein tyrosine kinase and T cell function. [15] However, Fei et al. concluded that nilotinib suppressed proliferation and function of Treg cells in a higher concentration in vitro (>10 μM), and was not shown to hamper the function of Treg cells at clinically relevant doses. [16] These findings, therefore, raise the possibility that the tyrosine kinase inhibitors, by blocking signaling in both Treg cells and effector T cells, could have an important pathogenic role in the development of psoriasis, however, the exact mechanism involved awaits further studies.
Patients with imatinib-aggravated psoriasis have shown improvement with etretinate, narrow band UVB, as well as methotrexate. [3] Since a significant response to topical therapy was not observed in our patient, methotrexate was initiated. Thus, while nilotinib is a newer effective chemotherapeutic agent for the treatment of chronic myeloid leukemia, optimal management of adverse effects requires prompt recognition and collaboration between dermatologists and oncologists.
| 1. |
Hu JC, Sadeghi P, Pinter-Brown LC, Yasher S, Chiu MW. Cutaneous side effects of epidermal growth factor receptor inhibitors: Clinical presentation, pathogenesis and management. J Am Acad Dermatol 2007;56:317-26.
[Google Scholar]
|
| 2. |
Brazzelli V, Grasso V, Borroni G. Imatinib, dasatinib, and nilotinib: A review of adverse cutaneous reactions with emphasis on our clinical experience. J Eur Acad Dermatol Venereol 2013;27:1471-80.
[Google Scholar]
|
| 3. |
Cheng H, Geist DE, Piperdi M, Virk R, Piperadi B. Management of imatinib-related exacerbation of psoriasis in a patient with a gastrointestinal stromal tumour. Australas J Dermatol 2009;50:41-3.
[Google Scholar]
|
| 4. |
Nagai T, Karakawa M, Komine M, Muroi K, Ohtsuki M, Ozawa K. Development of psoriasis in a patient with chronic myelogenous leukaemia during nilotinib treatment. Eur J Hematol 2013;91:270-2.
[Google Scholar]
|
| 5. |
Kantarjian H, Giles F, Wunderle L, Bhalla K, O′Brien S, Wassmann B, et al. Nilotinib in imatinib- resistant CML and Philadelphia chromosome- positive ALL. N Engl J Med 2006;354:2542-51.
[Google Scholar]
|
| 6. |
Yang JH, Lee DW, Won CH, Chang SE, Lee MW, Lee KH, et al. Rosacea like eruption associated with nilotinib. J Eur Acad Dermatol Venereol 2011;25:868-9.
[Google Scholar]
|
| 7. |
Drucker AM, Wu S, Busam KJ, Berman E, Amitay Laish I, Lacouture ME. Rash with the multitargeted kinase inhibitors nilotinib and dasatinib: Meta-analysis and clinical characterization. Eur J Haemtol 2013;90:142-50.
[Google Scholar]
|
| 8. |
Le Coutre P, Ottmann OG, Giles F, Kim DW, Cortes J, Gatterman N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood 2008;111:1834-9.
[Google Scholar]
|
| 9. |
Tasigna package insert. Basel, Switzerland: Novartis. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022068s004s005lbl.pdf.[Last accessed on 2014 Feb 8].
[Google Scholar]
|
| 10. |
Miyagawa S, Fujimoto H, Ko S, Hirota S, Kitamura Y. Improvement of psoriasis during imatinib therapy in a patient with a metastatic gastrointestinal tumor. Br J Dermatol 2002;147:406-7.
[Google Scholar]
|
| 11. |
Mattozzi C, Salvi M, D′Epiro S, Giancristoforos S, Macaluso L, Luci C, et al. Importance of regulatory T cells in the pathogenesis of psoriasis: Review of literature. Dermatology 2013;227:134-45.
[Google Scholar]
|
| 12. |
Larmonier N, Janilashvili N, LaCasse C, Larmonier CB, Cantrell J, Situ E, et al. Imatinib mesylate inhibits CD4+CD25+regulatory T cell activity and enhances active immunotherapy against BCR-ABL- tumors. J Immunol 2008;181:6955-63.
[Google Scholar]
|
| 13. |
Chen J, Schmitt A, Giannopoulos K, Chen B, Rojewski M, Dohner H, et al. Imatinib impairs the proliferation and function of CD4+CD25+regulatory T cells in a dose dependent manner. Int J Oncol 2007;31:1133-9.
[Google Scholar]
|
| 14. |
Chen J, Scmitt A, Chen B, Rojewski MT, Rubeler Y, Fei F, et al. Nilotinib hampers the proliferation and function of CD8+T lymphocytes through inhibition of T cell receptor signaling. J Cell Mol Med 2008;12:2107-18.
[Google Scholar]
|
| 15. |
Blake SJ, Lyons AB, Hughes TP. Nilotinib inhibits the Src-family kinase LCK and T cell function in vitro. J Cell Mol Med 2009;13:599-601.
[Google Scholar]
|
| 16. |
Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Greiner J, et al. Effects of nilotinib on regulatory T cells: The dose matters. Mol Cancer 2010;9:22.
[Google Scholar]
|
Fulltext Views
2,915
PDF downloads
1,270





