Translate this page into:
Niosomal methotrexate gel in the treatment of localized psoriasis: Phase I and phase II studies
2 Department of Pharmaceutical Marketing and Management, Al-Ameen College of Pharmacy, Hosur Road, Bangalore, India
3 Department of Pharmaceutics, Al-Ameen College of Pharmacy, Hosur Road, Bangalore, Karnataka, India
4 Department of Skin and STD, Victoria Hospital, Bangalore, Karnataka, India
Correspondence Address:
P K Lakshmi
Director-Drug Information, Karnataka State Pharmacy Council, 514/E, I main, II stage, Vijayanagar Club Road, Vijayanagar, Bangalore, Karnataka - 560 040
India
| How to cite this article: Lakshmi P K, Devi GS, Bhaskaran S, Sacchidanand S. Niosomal methotrexate gel in the treatment of localized psoriasis: Phase I and phase II studies. Indian J Dermatol Venereol Leprol 2007;73:157-161 |
Abstract
Background: Efficacy of topical methotrexate in psoriasis is limited by its penetration. Aims: The study involved the preparation of niosomal methotrexate in chitosan gel and to test the same for irritation and sensitization on healthy human volunteers followed by assessing the efficacy of the gel through double-blind placebo-controlled study on psoriasis patients and also comparing its efficacy with a marketed methotrexate gel. Methods: The methotrexate niosomes were prepared by lipid layer hydration method. The characterized niosomes were incorporated in chitosan gel. The gels were tested on 10 human volunteers to check for irritation and skin sensitivity by human repeated insult patch test (HRIPT). The formulations were assessed for efficacy by double-blind placebo-controlled study in 10 psoriasis patients for each formulation. The efficacy was calculated by psoriasis area and severity index scoring method. The global score was used to assess the progress of the disease. Results: The HRIPT test did not produce any significant irritation or sensitization on healthy human volunteers. The placebo and marketed gels were compared with niosomal methotrexate gel. At Week 12, with niosomal methotrexate gel, there was reduction in total score from 6.2378�1.4857 to 2.0023�0.1371. Conclusion: These results suggest that niosomal methotrexate gel is more efficacious than placebo and marketed methotrexate gel.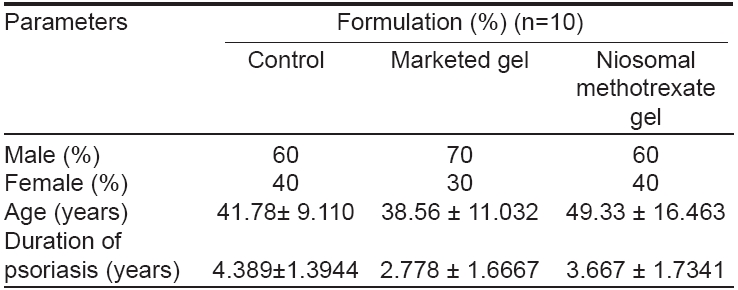

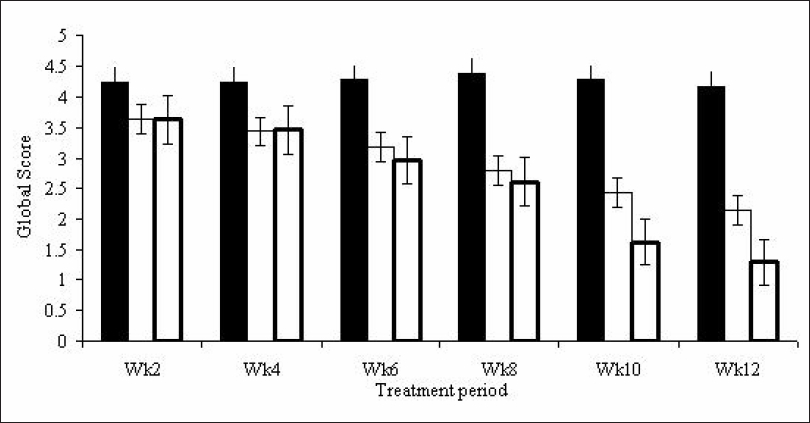 |
| Figure 2: Physician�s global score for the assessment of progress |
 |
| Figure 2: Physician�s global score for the assessment of progress |
 |
| Figure 1: Mean change (�SEM) in total PASI scores from baseline |
 |
| Figure 1: Mean change (�SEM) in total PASI scores from baseline |
Introduction
Niosomes or non-ionic surfactant vesicles are now widely studied as an alternative to liposomes. An increasing number of non-ionic surfactants have been found to form vesicles, capable of entrapping hydrophobic and hydrophilic solutes. These novel drug deliveries have been used in the preparation of various drugs to enhance the penetration and also to sustain the release of the drug. [1],[2]
The currently available topical treatments for psoriasis that have been found to be effective include coal tar, anthralin calcipotriol, corticosteroids, photochemotherapy, retinoids, methotrexate, urea etc. [3]
Methotrexate is an effective systemic cytotoxic drug for the treatment of extensive psoriasis. Unfortunately, the risk of hepatic fibrosis and cirrhosis has precluded the use of this drug in the great majority of patients with localized psoriasis. To avoid systemic toxicity, topical administration of methotrexate was attempted. Stewart et al and McCullough et al suggested that localized methotrexate did not yield much penetration to produce significant clinical effect in psoriasis. [4],[5] We tried a new approach for topical delivery of methotrexate by niosomal encapsulation that was aimed at increased percutaneous penetration and therapeutic effectiveness.
Methods
The phase I and phase II study, was conducted at Victoria Hospital, Bangalore during the period 2004-2005. Ethical clearance was obtained to conduct the trial on human volunteers and patients.
Preparation of test materials
The test materials used were niosomal methotrexate (0.25%) gel (CH1NMTX2), marketed methotrexate (0.25%) gel (MTX) and the placebo gel (control).
The lipid layer hydration method was followed with slight modifications. The niosomes formed were characterized for particle size, zeta potential, stability interaction with excipients and in vitro release studies. The characterized niosomes were incorporated in chitosan gel. Chitosan-I (mol. wt - 5.47x 10 5 D) and Chitosan-II (mol. wt - 8.82 x 10 5 D) were used in 2:1 proportion for the preparation of the gel. The gel was prepared by incorporating 0.25% equivalent of niosomal methotrexate in glycerin and propylene glycol. [6],[7],[8]
The pH of the marketed gel (Rextop© , Systopic India Limited TM ) was found to be 5.2 with viscosity of 7850 cps. The viscosity and pH of niosomal methotrexate was 8600 cps and 5.0-5.2 respectively.
The placebo, niosomal methotrexate gel and marketed gel were tested for repeated insult patch test (RIPT) on human volunteers to assess the irritation potential of the methotrexate gel on the skin. This was followed by a double-blind placebo-controlled study in patients of psoriasis to assess the efficacy. The permission to conduct a study was obtained from the institutional ethical committee (IEC).
The RIPT is a standard method to detect a product′s propensity to produce contact dermatitis. All the participating volunteers gave their informed consent before joining the study. Four female and six male healthy human volunteers with mean age of 35.5 ± 2.5 and 44 ± 1.5 respectively were selected for the study after the standard inclusion and exclusion criteria for RIPT were satisfied. The test was conducted in two phases, induction and challenge phase as per accepted protocol for such tests. [9],[10]
A small quantity of gel (0.2 g) was applied to the aluminum Finn chambers. The occlusive dressing used for covering the skin was ¾" x ¾" absorbent pad center of a non-porous adhesive dressing. The application area was scored on a 0-5 point scale as described by previous workers. [9],[10] In case a subject developed a Level 2 reaction or greater during the induction phase, the patch was applied to an adjacent fresh site for the next application. If a Level 2 or greater reaction occurred on the new site, no further induction applications were made. The human volunteers were given two weeks washout period and proceeded with the next formulation for RIPT.
Phase II study: Double-blind placebo-controlled study [11],[12]
The purpose of this placebo-controlled double-blind study was to evaluate the clinical efficacy and tolerability of niosomal methotrexate 0.25% incorporated chitosan gel to treat patients affected with localized psoriasis of less than 25% of body surface area involvement. All the participating patients gave their informed consent before joining the study. Thirty patients were randomized into three groups using computer-generated random numbers.
Adult patients of both sexes, suffering from stable psoriasis involving < 25% of the body surface area were included in the study. Pregnant and or lactating women and children were excluded. A washout period of two weeks for topical therapy and two months for systemic or ultraviolet therapy was observed. Patients with lesions on face or scalp alone were excluded. Alcoholic patients and those with concomitant renal, hepatic and hematological abnormalities were excluded.
Each patient underwent physical examination and standard laboratory tests such as complete hemogram, renal function, serum creatinine, blood urea nitrogen and liver function tests.
Patients were randomly allocated to three different groups. Study included twice daily application of 10 g gel over psoriatic lesions for five consecutive days per week except niosomal methotrexate which was applied once daily in the morning. Placebo gel was applied in the night. Patients were asked to apply not more than 0.5 g/day.
Drug-related side-effects reported by the patient were recorded by directly questioning the subjects. The efficacy of the treatment was evaluated at the end of every two weeks. The study was concluded at the end of 12 weeks.
The severity of the lesions of psoriasis was evaluated using Psoriasis Area Severity Index (PASI) described by Sutton et al and Frediksson et al . [11],[12] Global assessment was made by the physician to assess the outcome of therapy (on a scale of 0-5). [11] A score of 5 indicates the worsening of lesion and score 0 indicates complete clearance of lesion.
Statistical analysis
One-way ANOVA test was used to compare the relationship between the outcome and treatment with the help of Instat© statistical software. Tukey′s HSD test was used for post-hoc analysis. Variables such as age, sex and presence of plaque psoriasis and duration of the disease were examined to rule out differences between the control and the test formulations.
Results
The double-blind placebo-controlled study involved the assessment of efficacy of the test formulations on psoriasis. Full patient compliance was achieved with the study medication. The control and trial preparations were well tolerated by all the patients and there were no dropouts. All 30 patients were available for efficacy analysis. Demographically, control and test formulation groups were comparable in age, sex and the duration of the disease [Table - 1]. All patients completed the 12 weeks of active treatment.
The erythema scores suggest that there was no significant difference between the three groups at baseline. At the end of the second week both the active formulations showed significantly reduced erythema ( P < 0.05) as compared to placebo. From Week 6 there was significant ( P < 0.001) difference seen in the reduction of erythema by both the active formulations in comparison to placebo and also at that time, niosomal methotrexate was significantly better than the marketed formulation of methotrexate.
The infiltration scores at baseline were comparable in all three groups. Tukey′s test shows that the niosomal formulations were significantly different in reducing the infiltration scores compared to placebo and the marketed formulation. The niosomal formulation showed significant ( P < 0.001) difference from week 2 to week 12 suggesting that it was more efficacious than the placebo and the marketed formulation.
The desquamation scores at baseline suggested that there was no significant difference between the formulations. There was no difference in reduction of desquamation scores at week 2. However, from week 4 onwards there was a significant difference ( P < 0.001) in the reduction of desquamation scores between placebo and the niosomal formulation.
At baseline there was no significant difference in the PASI score between groups but from week 4 a significant difference ( P < 0.05) was seen between the placebo and the two active formulations. At week 6 and 8 there was a significant difference in reduction of PASI ( P < 0.05) with all the active formulations [Figure - 1]. At week 12, with niosomal methotrexate gel there was highly significant ( P < 0.001) reduction in total score from 6.7378±1.48576 to 2.0023±1.13718. These results suggested that niosomal methotrexate gel was more efficacious than placebo, marketed gel. From Week 4 to Week 12 there was a significant difference ( P < 0.001) in global score between the formulations [Figure - 2].
At the end of the study laboratory tests including complete blood count with differential and platelet count, renal function, serum creatinine, blood urea nitrogen and liver function test were found to be within normal limits. Patients experienced no drug-related adverse symptoms, either local or systemic.
Discussion
Systemic administration of methotrexate is known for its toxicity; hence the need for a topical preparation. Although earlier preparations of topical methotrexate were found to be not as efficacious, [4],[5] there is substantial evidence to support the pharmacological action of methotrexate in psoriasis. Methotrexate selectively inhibits de novo DNA synthesis, blocks mitosis and lethally damages cells in psoriatic epidermis when administered both systemically and locally by intradermal injection.
Studies done on methotrexate topical formulation in propylene glycol base, twice-daily application reported adequate percutaneous penetration and beneficial effect in palmoplantar psoriasis. Topical methotrexate in hydrophilic gel (hydroxy ethyl cellulose 1%) has been prepared and used in the treatment of psoriasis vulgaris. [13],[14] These studies concluded that methotrexate (0.25%) in a hydrophilic gel was clinically more effective, tolerable and beneficial in enhancing resolution of psoriasis vulgaris as compared to a placebo. [13],[14]
We attempted to reduce the systemic toxicity of methotrexate by encapsulating it in niosomes and providing the drug delivery through topical application. No single mechanism explains the efficacy of niosomes. Topically applied niosomes can increase the residence time of drugs in the stratum corneum (SC) and epidermis, while reducing the systemic absorption of the drug. They are thought to improve the horny layer properties, both by reducing trans-epidermal water loss and by increasing smoothness via replenishing lost skin lipids. The niosome vesicles form a lipid film on the skin, which can retain water, causing an improvement of SC intra- and intercellular hydration. Therefore, the compact structure of the SC is opened and the barrier permeability is increased. [2],[15]
For niosomal encapsulation, lipid layer hydration method with pH 5-phosphate buffer was used. The acid dissociation constant (pKa) of methotrexate is 5. At pH 5, methotrexate exists in unionized form. Topical application on the skin involves passive transport of drug and better transport was observed when in unionized form. Hence methotrexate in pH 5 buffer was used in the preparation of niosomes. The gels were prepared with chitosan. It provides absorption-promoting effect probably by improved adhesion between the formulation and the tissues, but also due to transient effect of chitosan on paracellular transport processes. [16],[17]
The current study comparing two formulations with placebo gel did not produce any significant irritation or sensitization on human volunteers as indicated by negative RIPT and these formulations were therefore later tested clinically on the psoriasis patients.
Though 20% patients treated with CH1NMTX2 and 40% patients treated with MMTX did not show much improvement, niosomal methotrexate 0.25% gel significantly improved psoriasis without inducing any allied side-effects. In comparison with the placebo and the marketed gel, results with niosomal preparation were much better in terms of clinical efficacy, tolerability and patient compliance.
The results show that topical methotrexate can treat psoriasis and niosomal methotrexate gel has better absorption and penetration of methotrexate through the skin. Once daily application of niosomal methotrexate gel may improve the compliance of psoriatic patients.
Though the results were encouraging, Phase II study was done on only 10 patients with each formulation in a single center. More number of patients with a multicentric study needs to be conducted to conclude about the efficacy of the new formulation.
Acknowledgment
We extend our thanks to Dabur India Limited and Fisheries department, Kerala for providing methotrexate USP, Spans and chitosan.
| 1. |
Yoshioka T, Brigitte Sternberg, Alexander T. Florence. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and sorbitan triester (Span 85). Int J Pharm 1994;105:1-6.
[Google Scholar]
|
| 2. |
Schreier H. Liposomes and niosomes as topical drug carriers: Dermal and transdermal delivery. J Control Release 1985;30: 863-8.
[Google Scholar]
|
| 3. |
Weinstein GD, McCullough JL, Olsen E. Topical methotrexate therapy for psoriasis. Arch Dermatol 1989;125:227-30.
[Google Scholar]
|
| 4. |
Stewart WD, Wallace SM, Runikis JO. Absorption and local action of methotrexate in human and mouse skin. Arch Dermatol 1972;106:357-61.
[Google Scholar]
|
| 5. |
McCullough JL, Synder DS, Weinstein GD, Friedland A, Stein B. Factors affecting human percutaneous penetration of methotrexate and its analogues in vitro . J Invest Dermatol 1976;66:103-7.
[Google Scholar]
|
| 6. |
Oommen E, Tiwari SB, Udupa N, Kamath R, Uma Devi P. Niosome entrapped b-cyclodextrin methotrexate complex as a drug delivery system. Indian J Pharmacol 1999;31:279-84.
[Google Scholar]
|
| 7. |
Idson B, Lazarus J. Semisolids. In : Lachman HA, Lieberman, Kanig JL, editors. The Theory and Practice of Industrial Pharmacy, 3 rd ed. Varghese Publishing House: Philadelphia; 1991. p. 534-63.
[Google Scholar]
|
| 8. |
Sankar V. Design and evaluation of nifedipine transdermal patches. J Pharm Sci 2003;65:510-5.
[Google Scholar]
|
| 9. |
Stots J. Planning, conduct and interpretation of human predictive sensitization patch tests. In : Drill VA, Lazar P, editors. Current concepts in cutaneous toxicity. Academic Press: New York; 1980. p. 41-53.
[Google Scholar]
|
| 10. |
Tardiff RG, Hubner RP, Graves CG. Harmonization of thresholds for primary skin irritation from results of human repeated insult patch tests and laboratory animal skin irritation tests. J Appl Toxicol 2003;23:279-81.
[Google Scholar]
|
| 11. |
Sutton L, Swinehart JM, Cato A, Kaplan AS. A clinical study to determine the efficacy and safety of 1% Methotrexate / azone (MAZ) gel applied topically once daily in patients with psoriasis vulgaris. Int J Dermatol 2001;40:464-7.
[Google Scholar]
|
| 12. |
Fredriksson T, Pettersson U. Severe psoriasis: Oral therapy with a new retinoid. Dermatologica 1978;157:238-44.
[Google Scholar]
|
| 13. |
Ravi Kumar BC, Inderjeet Kaur, Bhusan Kumar. Topical methotrexate therapy in palmoplantar psoriasis. Indian J Dermatol Venerol Leprol 1999;65:270-2.
[Google Scholar]
|
| 14. |
Syed TA, Hadi SM, Qureshi ZA, Nordstrom CG, Ali SM. Management of psoriasis vulgaris with methotrexate 0.25% in a hydrophilic gel: A placebo-controlled, double-blind study. J Cutan Med Surg 2001;5:299-302.
[Google Scholar]
|
| 15. |
Manconi M, Sinico C, Valenti D, Lai F, Fadda AM. A study into the in vitro cutaneous delivery of vesicle-incorporated tretinoin. Int J Pharm 2006;311:11-9.
[Google Scholar]
|
| 16. |
Singla AK, Chawla M. Chitosan: Some pharmaceutical and biological aspects - an update. J Pharm Pharmacol 2001;53:1047-67.
[Google Scholar]
|
| 17. |
Fang N, Chan V, Mao HQ, Leong KW. Interactions of phospholipid bilayer with chitosan: Effect of molecular weight and pH. Biomacromolecules 2001;2:1161-8.
[Google Scholar]
|
Fulltext Views
4,985
PDF downloads
1,655





