Translate this page into:
Nocebo effect in dermatology
2 University of Arkansas for Medical Sciences, Little Rock, AR, USA
3 Department of Dermatology and STD, UCMS and GTB Hospital, Delhi, India
4 Department of Psychiatry, Westmead Hospital, Sydney, NSW, Australia
5 Bhim Rao Ambedkar College (University of Delhi), Delhi, India
6 Center for Research and Training in Skin Diseases & Leprosy (CRTSDL), Tehran University of Medical Sciences, Tehran, Iran
7 Medic Affairs, Eli Lilly India Pvt Ltd., Gurgaon, India
Correspondence Address:
Sidharth Sonthalia
Skinnocence: The Skin Clinic, C-2246, Sushant Lok-1, Gurgaon
India
| How to cite this article: Sonthalia S, Sahaya K, Arora R, Singal A, Srivastava A, Wadhawan R, Zartab H, Gupta KS. Nocebo effect in dermatology. Indian J Dermatol Venereol Leprol 2015;81:242-250 |
Abstract
Nocebo effect, originally denoting the negative counterpart of the placebo phenomenon, is now better defined as the occurrence of adverse effects to a therapeutic intervention because the patient expects them to develop. More commonly encountered in patients with a past negative experience, this effect stems from highly active processes in the central nervous system, mediated by specific neurotransmitters and modulated by psychological mechanisms such as expectation and conditioning. The magnitude of nocebo effect in clinical medicine is being increasingly appreciated and its relevance encompasses clinical trials as well as clinical practice. Although there is hardly any reference to the term nocebo in dermatology articles, the phenomenon is encountered routinely by dermatologists. Dermatology patients are more susceptible to nocebo responses owing to the psychological concern from visibility of skin lesions and the chronicity, unpredictable course, lack of 'permanent cure' and frequent relapses of skin disorders. While finasteride remains the prototypical drug that displays a prominent nocebo effect in dermatologic therapeutics, other drugs such as isotretinoin are also likely inducers. This peculiar phenomenon has recently been appreciated in the modulation of itch perception and in controlled drug provocation tests in patients with a history of adverse drug reactions. Considering the conflict between patients' right to information about treatment related adverse effects and the likelihood of nocebo effect stemming from information disclosure, the prospect of ethically minimizing nocebo effect remains daunting. In this article, we review the concept of nocebo effect, its postulated mechanism, relevance in clinical dermatology and techniques to prevent it from becoming a barrier to effective patient management.INTRODUCTION
Placebo (latin, ′I shall please′) is a very well appreciated effect in both clinical trials and practice. Physicians often bring up the "placebo effect" to explain the unexpected self-perceived improvement reported by patients in response to a therapy. Apparently, the first placebo-controlled clinical trial assessing the efficacy of metallic rods called Perkins tractors, as a therapeutic tool was performed at the turn of the 18 th century. [1] The term ′placebo′ made its debut in 1938, in a double-blind clinical trial by Diehl et al., to assess the efficacy of cold vaccines amongst the students of University of Minnesota. [2] Henceforth, ′placebo-controlled′ trials came into being.
Nocebo (latin, ′I shall harm′) effect on the other hand is a relatively novel and less appreciated concept. It pertains to the occurrence of adverse effects because they are expected to develop, attributed to the intervention. [3],[4],[5] Patients often report poor tolerance to one or many of the prescribed medications. Similar to the placebo effect, nocebo effects are also encountered in both clinical trials and practice. The effects can be non-specific, subjective, objective or specific to the intervention. [3],[6],[7] Experience with placebo-controlled trials has confirmed that patients receiving placebo often report side effects similar to those experienced by patients in the drug group. These side effects, result from mere disclosure of potential adverse effects during informed consent. [3],[5] Nocebo effect also has a significant bearing on healthcare costs. [8] In this review, we discuss the nocebo effect, postulated pathophysiological mechanisms, its potential and magnitude in clinical medicine with special emphasis on dermatology.
Terminology and history
The term "nocebo effect" was originally coined by Kennedy (1961) to denote the negative counterpart of the placebo phenomenon. [4] Colloca and Miller have used the term "nocebo" to indicate an inert substance or procedure intended to create negative expectations, typically for experimentation or clinical trial. [5] The terms ′nocebo effect′ and "nocebo response", albeit similar, also need to be differentiated. The pessimistic psychosocial context around the patient associated with a drug or intervention is designated ′nocebo effect′. However, ′nocebo response′ better indicates the expectancy-induced changes in the patient′s brain-body unit with consequent worsening of patients′ outcome, induced by the words and elements of the clinical encounter, without administration of an inert substance. [5] As practitioners, we often promote placebo responses to our prescribed drugs by instilling confidence in the patient about its efficacy. Logically, physicians would not intentionally produce nocebo responses; it being unethical and averse to the basic healthcare concept of benefaction. The sole exception is the use of nocebo for ′nocebo-controlled′ drug challenge testing in patients with history of adverse drug reactions (ADRs). [5]
Mechanism of nocebo
Latest scientific evidence indicates that these effects stem from highly active processes in the central nervous system (CNS), mediated by psychological mechanisms such as expectation and conditioning.
Neurophysiological pathways involved in nocebo effect
The pain model has served as the pedestal for study of mechanisms underlying these effects [Figure - 1]. [9] Their effects in non-pain-related conditions have been explored only recently. [10],[11],[12] Nocebo response requires the interaction of a stimulus with conscious or subconscious expectation of a negative response, which may occur at cortical, subcortical, and/or spinal level. [9],[13],[14] For example, when an inert cream suggested to contain capsaicin was applied over the forearm of a human volunteer, evaluation by functional magnetic resonance imaging revealed lowered pain threshold and increased pain perception. [15]
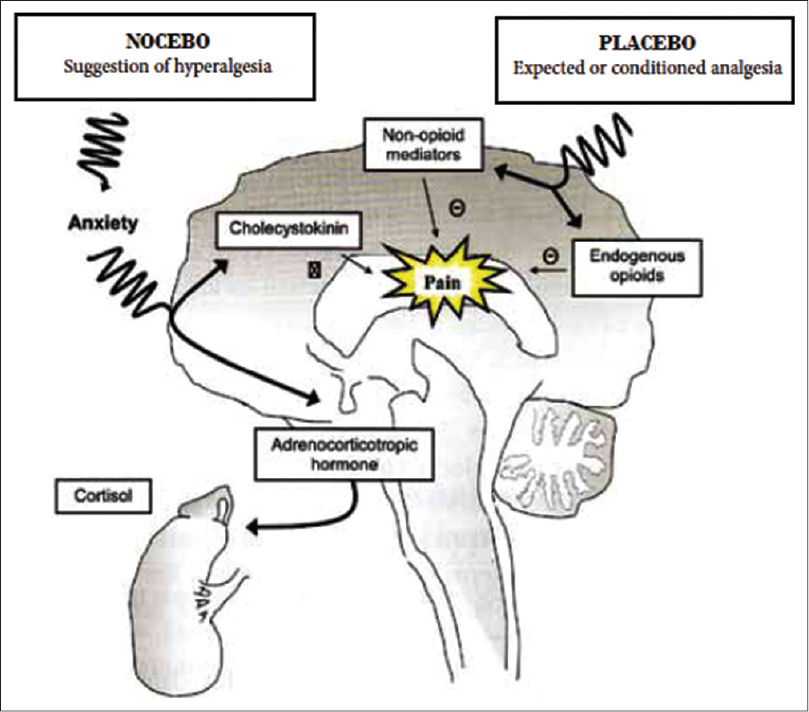 |
| Figure 1: Postulated neurophysiological mechanism of the hyperalgesic nocebo response |
Placebo-induced analgesia seems to be initiated in the pre-frontal cortex with top-to-down modulation involving the rostral anterior-cingulate cortex and peri-aqueductal gray followed by recruitment of opioid dependent descending pain modulatory pathways. [14] The placebo and nocebo responses seem to share some common pathways with reciprocal responses such as the dopaminergic and opioid activity in nucleus accumbens, activation with placebo, and deactivation with nocebo. [16] The nocebo responses additionally seem to involve distinct pathways involving the hippocampus and para-hippocampal formations. [9]
Role of neurotransmitters
Expectedly, the neurotransmitters implicated in the genesis of placebo and nocebo effects are physiologically opposing; endogenous opioids and cholecystokinin seem to be the major neurotransmitters involved in the generation of placebo and nocebo responses, respectively. [13],[16],[17],[18] In early experiments, administration of naloxone (an opioid antagonist) blocked placebo response to pain. Nocebo is more closely modulated by cholecystokinin. In two studies, the non-specific cholecystokinin antagonist proglumide blocked the nocebo effect in postoperative patients as well as healthy volunteers. [17],[19] Moreover dopaminergic activation in the ventral basal ganglia contributes to placebo and deactivates nocebo responses. [16] Role of behavioral state and other modifiers on the nocebo response is also undeniable. In healthy volunteers, diazepam was able to block the nocebo response. [16] These curious responses are not entirely dependent on conscious perception of cues since they may be generated by unconscious cues as well. [20]
Psychological enhancement of nocebo
The basic psychological mechanisms underlying nocebo responses are: (i) information about negative outcomes, given by physicians or gathered from sources including other patients and internet; (ii) past experience of negative therapeutic outcomes, and; (iii) observation of other patients′ negative outcomes. Nocebo responses are more commonly encountered in a "difficult" patient, defined as one who impedes the clinician′s ability to establish a therapeutic relationship. [21] Although a prior negative patient experience with physicians constitutes the commonest cause for a difficult patient encounter, specific psychological disorders also contribute, including somatoform disorders, personality disorders, and adjustment disorders, with associated feelings of guilt and/or obsession. [21]
Expectation can lead to attention being focussed on such symptoms and hence, higher chances of reporting adverse effects. Mis-attribution of unrelated or pre-existing symptoms to a medication is common in people experiencing higher levels of negative emotions or stressful situations. [22] Another interesting example is the perceived reduced efficacy and greater adverse effects with generic drugs compared to branded drugs. A recent study with placebo, branded and generic drugs showed how negative expectations led to reduced efficacy and increased side effects. [23]
Magnitude and relevance in clinical medicine
Nocebo effect in clinical trials
The nocebo effect is widely prevalent in clinical trials. Participants in the placeboarm of trials often report adverse effects to the sham intervention. The percentage of reported adverse effects in the placeboarm has been as high as 64-74% in studies on neurological disorders. [24],[25] A common nocebo effect-related problem in clinical trials is the withdrawal by research subjects. In a pooled analysis of neuropathic pain trials, 6% of patients dropped out due to non-specific nocebo-related adverse effects. [26] Anticipation stemming from their mention in the trial consent form may result in increased reporting of adverse effects by the placeboarm. [27] Researchers distinguish between ′apparent′ and ′true′ placebo effects. The apparent placebo effect may be due to factors such as the natural history of the condition under study. On the contrary, a true placebo effect is attributed to the placebo intervention. A similar distinction cannot be extrapolated to nocebo effect. [5] Apparent nocebo effects are adverse responses observed in the placeboarm of a randomized controlled trial. [5] Contrarily, true nocebo effect in double-blind drug trials includes all negative effects in placebo groups minus non-specific factors such as symptoms from the treated disease or comorbid conditions, and adverse events of accompanying medication. [28]
Nocebo effect in clinical practice
" To tell the truth, the whole truth, may do patients harm!" [29] In day-to-day practice, nocebo effects result from interactions between physician and patient, patient and patient, and the general psychosocial context surrounding the patient and intervention. Nocebo reactions are observed more often in women and older people. Examples of nocebo effect encountered in practice include gastrointestinal adverse effects to antibiotics and hematinics, drowsiness to non-sedating antihistamines and erectile dysfunction (ED) with beta-blockers, among others.
Nocebo effect, informed consent and ethics
Physicians are obligated to convey truthful information to patients to help them make informed decisions about their treatment. However, information disclosure may itself contribute to negative expectations and nocebo effects. With such suspicion leading to discontinuation of an otherwise effective drug, should the release of such information be limited? This sensitive issue has a direct bearing on concepts of informed consent, patient autonomy, and safety. Traditionally, physicians have condescendingly restricted the disclosure of information to patients. However, the dogma of current healthcare delivery, medical ethics and law endorse the concept of informed consent as the patients′ "therapeutic privilege". [30] The dual challenge of honoring the patient′s right to information, yet minimizing the possibility of nocebo responses is indeed daunting for today′s physicians.
In our opinion, it is imperative for the health care provider to give information about possible life-threatening and other serious adverse effects of the prescribed medication. Whether every patient should be informed in detail about the possibility of such reactions is a gray area of therapeutic counseling with obvious contradictions in formulating such guidelines and recommendations. Information regarding dose-related and other non-specific adverse effects is equally relevant, for example, warning about drowsiness due to sedative antihistamines and counseling about precautions to be taken while driving or working with heavy machinery to prevent an occupational mishap or injurious fall. Altering the way of communicating about possible drug adverse effects, thereby minimizing negative instructions and negative therapeutic contexts has been suggested as the most effective way of mitigating the nocebo effect (vide infra). [29],[31]
Nocebo effect in dermatology
What the thinker thinks, the prover proves! And skin diseases are no different. Despite negligible reference to the term nocebo in dermatology publications, the phenomenon is routinely encountered by dermatologists. Dermatoses such as psoriasis, vitiligo, and eczemas are typecast by chronicity, unpredictable course, lack of ′permanent cure′ and frequent relapses. Moreover, skin diseases usually being visible to others have an immense psychological impact. Compared to healthy controls, anxiety levels, risk of depression, and impaired quality of life (QoL) are significantly higher in patients with skin disorders resulting in increased vulnerability to nocebo responses. [32],[33] The additional influence of information technology like internet-based search engines and online discussion fora is worth mentioning. These sites, together with social media portals (Facebook and Twitter) have facilitated the spread of ′mass psychological illness′ symptoms. [34] The plethora of uncontrolled information on the internet affects one′s perspective about a drug.
Nocebo effect with finasteride
Finasteride is the classic example of a drug used in dermatology and urology with significant nocebo responses. Male pattern baldness (MPB) is androgen dependent with evidence-based efficacy of low dose finasteride (1 mg/day), an antiandrogen that blocks conversion of testosterone to dihydrotestosterone (DHT). This drug, used for years by urologists for benign prostatic hyperplasia (BPH) is infamous for its association with sexual dysfunction. Androgens, especially testosterone increase libido. Any drug that interferes with the action of androgens is therefore assumed by the lay man to decrease libido and cause impotence. [35] Sexual adverse effects of finasteride are well publicised and are often already known to the patients when they visit the dermatologist. Incidence of sexual adverse effects has varied significantly in different studies. A comprehensive review of 73 papers on medical therapies for benign prostatic hyperplasia revealed that finasteride is only infrequently associated with sexual adverse effects, with erectile dysfunction being the most common (4.9-15.8%) followed by ejaculatory problems (2.1-7.7%) and decreased libido (3.1-5.4%). [36] This is in contrast to the higher percentage of patients in clinical practice who are anxious about or complain of these side effects. Could nocebo effect be the cause? Let′s ponder over the following facts: androgens are not the only driving force behind libido; visual, olfactory, tactile, auditory, and imaginative stimuli also influence it. Erection is not exclusively dependent on testosterone levels. Penile erection is mainly under the control of parasympathetic nervous system whereas ejaculation and detumescence depend on an intact sympathetic system. [35],[37]
Decreased ejaculatory volume because of predominant action of dihydrotestosterone on prostate has been reported to be the only sexual adverse effect with a causal relation to finasteride. [38]
In many studies, incidence of sexual adverse effects with finasteride has been comparable to placebo. There is lack of evidence favoring a clear association of sexual adverse effects with dose or duration of therapy. [39],[40]
Patients who are informed about the reported sexual adverse effects of finasteride in detail initially complain of multiple sexual adverse effects occurring at the same time, that too within the first week of starting the treatment (personal observation). This undoubtedly fails to stand up to scientific scrutiny. Furthermore, after a single convincing counseling session, patients report disappearance of the sexual adverse effects within a few days despite continuing finasteride.
More convincingly, in a recent study by Mondaini et al. in patients with benign prostatic hyperplasia, blinded administration of 5 mg finasteride was associated with a significantly higher proportion of sexual dysfunction in patients informed about sexual adverse effects, compared with those who were uninformed. [41]
Based on the above, sexual adverse effects due to finasteride seem to have a strong component of nocebo response. Certain studies have documented contrary findings, particularly with respect to the ′post-finasteride syndrome′. [42],[43],[44] This syndrome, characterized by sexual and certain non-sexual adverse effects experienced at least 3 months after stopping the medication and persisting indefinitely, has generated a commotion in the scientific community. However, the existing evidence from systematic surveys and anecdotal observations is not robust enough to prove or disprove the biological plausibility of this syndrome.
In view of the conflicting data and continuing importance of the subject, the International Society of Hair Restoration Surgery (ISHRS) established a "Task Force on Finasteride Adverse Event Controversies" to evaluate published data and make recommendations. The latest update on this issue posted at their official website is detailed in Box 1. [45]

Pending a settling conclusion to the causal association between finasteride and sexual adverse effects, the prospect of drug-induced impotence remains appalling for the lay man. Skewed information derived from websites and blogs further complicate the situation.
Nocebo and itch
Itch, a common symptom of many dermatoses and systemic diseases, becomes irksome as it attains chronicity. The phenomenon of "contagious" itch, that is, inducement of a sensation of itch in the observer after watching someone scratch himself, indicates the susceptibility of itch to suggestion. [46],[47] The impact of verbal suggestion-induced nocebo and possibly placebo effects on itch has been confirmed earlier. [11] In one study, histamine-induced reactions were stronger in patients with atopic dermatitis who had been primed with nocebo-related itch suggestions. [46] In a recent study by Bartels et al., who investigated the role of verbal suggestion and conditioning in placebo and nocebo effects on itch, the subjects receiving both conditioning and verbal suggestion experienced significant nocebo effects, that is, subjects experienced a lot more itch when they were verbally suggested and conditioned with visual cues that the stimulus would evoke more itch than when given neutral suggestions (control group). [12] Another interesting finding of this study was the statistically significant association of nocebo effect with individual psychological characteristics of hope, extraversion, negative affect, and worrying. Greater nocebo responses were observed with more worry and negative effect, less hope, and lower levels of extraversion. These newer insights of the impact of nocebo-placebo effects on itch are expected to translate into novel therapeutic approaches for chronic itch based on reducing unfavorable expectations and enhancing favorable expectations.
Nocebo effect with isotretinoin
Oral isotretinoin is a widely used drug in dermatology with indications expanding beyond acne and psoriasis. Isotretinoin is well known to be associated with a spectrum of adverse reactions including teratogenicity, cutaneous eruptions, hepatitis, pancreatitis and ocular changes. Owing to the plethora of side effects, patients are usually informed about a few common adverse effects before starting the treatment. Patients are also advised to inform the treating physician about specific symptoms/adverse effects such as severe headache, excessive lethargy and depression. Women in the reproductive age group are briefed about specific contraception-related instructions. Expectedly, patients end up exploring the internet to access more information about isotretinoin, making them vulnerable to the development of nocebo effects. Despite this logical expectation, significant isotretinoin-related adverse effects have not been reported in the placebo arm of controlled studies conducted in acne patients. However, some evidence suggestive of a nocebo effect from isotretinoin has accrued from non-acne based studies. In a multicentric double-blinded trial, 573 patients with rosacea grade II/III were randomized to receive oral isotretinoin, doxycycline, or placebo for 12 weeks. [48] All patients were informed in detail about the possible adverse effects of study drugs. Interestingly, 30% and 17% patients in the placebo group and 24% patients treated with doxycycline, also reported xerosis cutis and cheilitis, respectively, even though these isotretinoin-associated side effects are unknown for doxycycline. [48] In another randomized controlled trial evaluating the role of low-dose daily oral isotretinoin (10 mg/day) given over 3 years for cancer chemoprevention, 29% of patients in the placebo group reported isotretinoin-related adverse effects and 11% permanently discontinued treatment due to putative adverse effects. [49] It is likely, although not absolutely certain that such a high incidence of typical isotretinoin-related adverse effects noted in the non- isotretinoin arms of these trials represents a nocebo effect.
Drug allergy and ′nocebo-controlled′ trials
Oral drug rechallenge is a well-known procedure for confirming adverse drug reactions. However, nocebo responses from patients′ previous experiences often influence the subjective perception of symptoms on re-exposure. To discern whether the symptoms during drug provocation represent true adverse effects or false positive responses, ′placebo-controlled′ oral challenge is considered the standard approach. Some studies have focused on evaluation of the nocebo effect in such blinded drug challenge tests. In a multicentric trial by Liccardi et al., the occurrence and clinical characteristics of nocebo effect in patients with adverse drug reactions were evaluated. Out of the 600 patients with a history of adverse drug reactions who underwent a blind oral challenge with the administration of an inert substance (called ′placebo′ in this trial) and active drugs, untoward reactions were provoked in 27% patients who were administered ′placebo′. [50] Although the majority of reactions were subjective (itching, malaise, headache, etc.,) 5% of patients showed cutaneous manifestations such as erythema, rash, and urticaria. In another drug challenge study by Lombardi et al., nocebo effect in the form of subjective symptoms (malaise, itching, abdominal pain) were observed in 13 (3%) of the 435 patients with history of adverse drug reactions. [51] Additionally, 10 out of these 13 patients had an abnormal result on the hospital anxiety depression questionnaire. An appraisal of risk factors for nocebo effect in drug challenge was conducted in a recent case-control study that included 228 patients with history of adverse drug reactions; 137 subjects (who reacted to provocation with an inert ′placebo′) and 91 controls (who did not show any reaction to ′placebo′). [52] Most nocebo responses (71.5%) were classified as subjective, with local pruritus being the most common. A minority (11.7%) were objective, with flushing and urticaria topping the list. Subjects with higher level of education, non-atopy, and longer history of drug hypersensitivity reactions were found to be more likely to experience the nocebo effect. [52] Thus, during oral drug provocation, the knowledge of this phenomenon is particularly significant for the treating physician to detect false positive responses that may misguide future treatment strategy. We believe that the term ′nocebo-controlled′ should be preferred over ′placebo-controlled′ in this context, since the inert substance, which is given as control, better qualifies for the term ′nocebo′.
Distinguishing true adverse drug effect from nocebo effect
The crux of the entire concept of nocebo effect revolves around two goals: Ascertaining whether a reported side-effect is a true adverse effect or due to the nocebo phenomenon, and employment of strategies to alleviate the nocebo effect in clinical practice. Keeping a high index of clinical suspicion based on certain pointers may aid the clinician. The consideration of a reported adverse effect should skew toward the possibility of being a nocebo effect in:
- A ′difficult patient′ (vide supra), that is, a patient with prior negative patient experience with physicians, patient with negative a effect, less hope with expectations of adverse outcome, and one who tends to somatize
- Patients with preconceived ideas about the adverse effects of the drug, most commonly generated through interaction with family members or friends and strong resistance to prescription of that drug despite detailed counseling by the clinician
- A patient who reports adverse effects based on his knowledge from the drug information sheet or the internet
- A patient who reports the particular adverse effect after having started the drug at a point which is inconsistent with the expected time frame.
Of course, this distinction has to be made on a case-to-case basis and despite harboring a strong suspicion of nocebo effect in an odd case, the clinician should be aware that reported adverse effect may be true.
Management strategies to alleviate nocebo effect in clinical practice
The impasse to the dilemma of obtaining informed consent and still minimizing nocebo-related risks rests in identifying nocebo-prone ′difficult′ patients and individualizing the method of disclosing information [Box 2]. The framework of revealing truthful information relating to side effects of treatments has various facets:
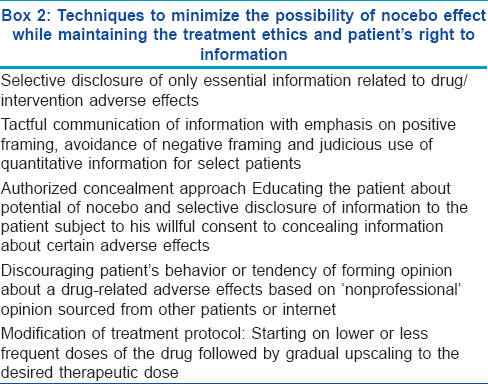
Amount of information to be disclosed: Information about certain drug-specific life threatening adverse effects and clues to self-identify them should be disclosed. Similarly, side effects with significant impact on quality of life should also be revealed but preferably with positive framing (vide infra). Informing the patient about every possible minor and major adverse effect of the drug is clearly unwarranted
Communication technique: The probability of experiencing adverse effects, based on contemporary research, can be communicated qualitatively and/or quantitatively (or statistically). For example, a non-serious side effect such as skin dryness or irritation can be mentioned simply as a minor possibility. [5] Such an attempt to trivialize its possible occurrence may help to avert a nocebo response
Qualitative information may be conveyed "negatively" (by focusing on the minority of patients who experience a particular side effect) or "positively" (by focusing on most patients who do not experience the side effect); as suggested by Colloca and Miller. [5] A positive framing is logically expected to downplay nocebo responses. Reassurance regarding reversibility of adverse effects on cessation of the drug (wherever applicable) may help in achieving this goal. Mentioning that a particular adverse effect has not yet been causally linked to the suspected drug may also comfort a skeptical patient
Quantitative or statistical information from published scientific data is more appealing for patients with a higher level of education. For example, likelihood of sexual adverse effects with oral finasteride for benign prostatic hyperplasia is around 5%. Indicating a lesser possibility of sexual adverse effects with its lower dose in male pattern baldness will instill more confidence in the patient. The positive effect of combining quantitative information with positive framing was demonstrated in a study evaluating adverse effects and work absenteeism after influenza immunization. Patients who were apprised about the percentage of patients free of vaccine side effects (positive framing) reportedly had significantly lesser adverse effects than patients who were informed about their occurrence (negative framing) [53]
Educating about potential of nocebo - the authorized concealment approach: To downplay nocebo effects consistent with patient autonomy, a technique of "authorized concealment" is worth discussing. In this approach, patients are asked if they are willing to agree not to receive information about certain side effects of the prescribed drug. [5] Serious or irreversibly harmful adverse effects should not be concealed. Authorized concealment may be appropriate for relatively mild and/or transient side effects. Patient′s may be informed about the possibility of bothersome but not life-threatening adverse effects happening in a small proportion of patients. [5] The biggest dilemma of this approach is ′what to conceal and what to reveal′, as the concealed side effects might be very relevant for some patients. The information about sexual adverse effects of finasteride for male pattern baldness epitomizes this predicament. However, overtones regarding ethicality of this approach need fine tuning
Discouraging ′non-professional′ opinion seeking behavior: As outlined above, a great deal of the nocebo effect is contributed by solicited feedback taken from other patients and internet portals. Patients should be cautioned against forming their opinion based on such grapevine information that lacks scientific evidence,
Modification of treatment protocol: Administration of lower daily doses or staggered pulsed doses of the drug, followed by subsequent upscaling of the dose may enhance patient compliance and abrogate the nocebo response. [35] This has been found to be effective for finasteride as well as isotretinoin. Finasteride doses as low as 0.2 mg/day adequately suppress both scalp skin and serum dihydrotestosterone levels, with clinical efficacy comparable to higher daily doses of 1 and 5 mg. [54] Similarly, low dose isotretinoin has been reported to be equi-efficacious to higher doses in multiple acne trials. [55] To vitiate the possibility of nocebo effect, isotretinoin may be initially administered at a lower daily dose (0.3 mg/kg) or on alternate days, followed by the full therapeutic dose (0.5-1 mg/kg/day), once the patient is comfortable. The value of similar regimens has been proven in a preliminary study. [56]
Patient counseling with the goal of minimizing nocebo effect is a continuous process requiring reinforcements during subsequent patient-physician encounters. Physician has to be careful to in discriminate the nocebo effect from an impending ′true′ adverse effect. A true adverse effect misconstrued as nocebo effect may be life-threatening for the patient, and harmful to the physician′s reputation. Timely detection of a true adverse effect, serious or mild, and its efficient management are paramount for good and safe clinical practice.
CONCLUSION
In conclusion, nocebo effect is an often overlooked phenomenon, a strong barrier to patient compliance, with the potential of depriving a patient of optimum treatment. It is time for dermatologists to appreciate its magnitude and impact and to consider and innovate approaches to circumvent its effect on their patients. Promotion of a healthier patient-physician communication and timely identification and effective management of symptoms hold the key to this goal. Future empirical research is exigent to assess the nature and impact of nocebo effect with other dermatological therapeutics and to develop innovative, safe, and ethical strategies to deal with it.
| 1. |
de Craen AJ, Kaptchuk TJ, Tijssen JG, Kleijnen J. Placebos and placebo effects in medicine: Historical overview. J R Soc Med 1999;92:511-5.
[Google Scholar]
|
| 2. |
Waller LA. A note on Harold S. Diehl, randomization, and clinical trials. Control Clin Trials 1997;18:180-3.
[Google Scholar]
|
| 3. |
Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. JAMA 2002;287:622-7.
[Google Scholar]
|
| 4. |
Kennedy WP. The nocebo reaction. Med World 1961;95:203-5.
[Google Scholar]
|
| 5. |
Colloca L, Miller FG. The nocebo effect and its relevance for clinical practice. Psychosom Med 2011;73:598-603.
[Google Scholar]
|
| 6. |
Rosenzweig P, Brohier S, Zipfel A. The placebo effect in healthy volunteers: Influence of experimental conditions on physiological parameters during phase I studies. Br J Clin Pharmacol 1995;39:657-64.
[Google Scholar]
|
| 7. |
Keitel A, Ferrea S, Südmeyer M, Schnitzler A, Wojtecki L. Expectation modulates the effect of deep brain stimulation on motor and cognitive function in tremor-dominant Parkinson's disease. PLoS One 2013;8:e81878.
[Google Scholar]
|
| 8. |
Gyllensten H, Rehnberg C, Jönsson AK, Petzold M, Carlsten A, Andersson SK. Cost of illness of patient-reported adverse drug events: A population-based cross-sectional survey. BMJ Open 2013;3 (6).pii: e002574.
[Google Scholar]
|
| 9. |
Tracey I. Getting the pain you expect: Mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med 2010;16:1277-83.
[Google Scholar]
|
| 10. |
Goebel MU, Meykadeh N, Kou W, Schedlowski M, Hengge UR. Behavioral conditioning of antihistamine effects in patients with allergic rhinitis. Psychother Psychosom 2008;77:227-34.
[Google Scholar]
|
| 11. |
van Laarhoven AI, Vogelaar ML, Wilder-Smith OH, van Riel PL, van de Kerkhof PC, Kraaimaat FW, et al. Induction of nocebo and placebo effects on itch and pain by verbal suggestions. Pain 2011;152:1486-94.
[Google Scholar]
|
| 12. |
Bartels DJ, van Laarhoven AI, Haverkamp EA, Wilder-Smith OH, Donders AR, van Middendorp H, et al. Role of conditioning and verbal suggestion in placebo and nocebo effects on itch. PLoS One 2014;9:e91727.
[Google Scholar]
|
| 13. |
Eknoyan D, Hurley RA, Taber KH. The neurobiology of placebo and nocebo: How expectations influence treatment outcomes. J Neuropsychiatry Clin Neurosci 2013;25:vi-254.
[Google Scholar]
|
| 14. |
Bingel U, Tracey I, Wiech K. Neuroimaging as a tool to investigate how cognitive factors influence analgesic drug outcomes. Neurosci Lett 2012;29;520:149-55.
[Google Scholar]
|
| 15. |
Geuter S, Büchel C. Facilitation of pain in the human spinal cord by nocebo treatment. J Neurosci 2013;33:13784-90.
[Google Scholar]
|
| 16. |
Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry 2008;65:220-31.
[Google Scholar]
|
| 17. |
Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci 2006;26:12014-22.
[Google Scholar]
|
| 18. |
Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet 1978;2:654-7.
[Google Scholar]
|
| 19. |
Benedetti F, Amanzio M, Casadio C, Oliaro A, Maggi G. Blockade of nocebo hyperalgesia by the cholecystokinin antagonist proglumide. Pain 1997;71:135-40.
[Google Scholar]
|
| 20. |
Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, et al. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci U S A 2012;109:15959-64.
[Google Scholar]
|
| 21. |
Trueb RM. The difficult hair loss patient. A particular challenge. Int J Trichol 2013;5:110-4.
[Google Scholar]
|
| 22. |
Faasse K, Petrie KJ. The Nocebo Effect: Patient expectations and medication side effects. Postgrad Med J 2013;89:540-6.
[Google Scholar]
|
| 23. |
Faasse K, Cundy T, Gamble G, Petrie KJ. The effect of an apparent change to a branded or generic medication on drug effectiveness and side effects. Psychosom Med 2013;75:90-6.
[Google Scholar]
|
| 24. |
Mitsikostas DD. Nocebo in headaches: Implications for clinical practice and trial design. Curr Neurol Neurosci Rep 2012;12:132-7.
[Google Scholar]
|
| 25. |
Stathis P, Smpiliris M, Konitsiotis S, Mitsikostas DD. Nocebo as a potential confounding factor in clinical trials for Parkinson's disease treatment: A meta-analysis. Eur J Neurol 2013;20:527-33.
[Google Scholar]
|
| 26. |
Papadopoulos D, Mitsikostas DD. A meta-analytic approach to estimating nocebo effects in neuropathic pain trials. J Neurol 2012;259:436-47.
[Google Scholar]
|
| 27. |
Myers MG, Cairns JA, Singer J. The consent form as a possible cause of side effects. Clin Pharmacol Ther 1987;42:250-3.
[Google Scholar]
|
| 28. |
Häuser W, Hansen E, Enck P. Nocebo phenomena in medicine: Their relevance in everyday clinical practice. Dtsch Arztebl Int 2012;109:459-65.
[Google Scholar]
|
| 29. |
Wells RE, Kaptchuk TJ. To tell the truth, the whole truth, may do patients harm: The problem of the nocebo effect for informed consent. Am J Bioeth 2012;12:22-9.
[Google Scholar]
|
| 30. |
Berg JW, Appelbaum PS, Lidz CW, Parker LS. Informed Consent: Legal theory and clinical practice. 2 nd ed. New York, NY: Oxford University Press; 2001.
[Google Scholar]
|
| 31. |
Colloca L, Finniss D. Nocebo effects, patient-clinician communication, and therapeutic outcomes. JAMA 2012;307:567-8.
[Google Scholar]
|
| 32. |
Cömert A, Akbaþ B, Kýlýç EZ, Akýn Ö, Gökçe E, Göktuna Z, et al . Psychiatric comorbidities and alexithymia in patients with seborrheic dermatitis: A questionnaire study in Turkey. Am J Clin Dermatol 2013;14:335-42.
[Google Scholar]
|
| 33. |
Chrostowska-Plak D, Reich A, Szepietowski JC. Relationship between itch and psychological status of patients with atopic dermatitis. J Eur Acad Dermatol Venereol 2013;27:e239-42.
[Google Scholar]
|
| 34. |
Bartholomew RE, Wessely S, Rubin GJ. Mass psychogenic illness and the social network: Is it changing the pattern of outbreaks? J R Soc Med 2012;105:509-12.
[Google Scholar]
|
| 35. |
Mysore. Finasteride and sexual side effects. Indian Dermatol Online J 2012;3:62-5.
[Google Scholar]
|
| 36. |
Carbone DJ Jr, Hodges S. Medical therapy for benign prostatic hyperplasia: Sexual dysfunction and impact on quality of life. Int J Impot Res 2003;15:299-306.
[Google Scholar]
|
| 37. |
Sawaya ME. Antiandrogens and androgen inhibitors. In: Wolverton SE, editor. Comprehensive Dermatologic drug therapy. 2 nd ed. Philadelphia: Saunders; 2007. p. 417-35.
[Google Scholar]
|
| 38. |
Erdemir F, Harbin A, Hellstorm WJ. 5á reductase inhibitors and erectile dysfunction: The connection. J Sex Med 2008;5:2917- 24.
[Google Scholar]
|
| 39. |
Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol 1999;41:555-63.
[Google Scholar]
|
| 40. |
Leyden J, Dunlap F, Miller B, Winters P, Lebwohl M, Hecker D, et al. Finasteride in the treatment of men with frontal male pattern hair loss. J Am Acad Dermaol 1999;40:930-7.
[Google Scholar]
|
| 41. |
Mondaini N, Gontero P, Giubilei G, Lombardi G, Cai T, Gavazzi A, et al. Finasteride 5 mg and sexual side effects: How many of these are related to a nocebo phenomenon? J Sex Med 2007;4:1708-12.
[Google Scholar]
|
| 42. |
Melcangi RC, Caruso D, Abbiati F, Giatti S, Calabrese D, Piazza F, et al. Neuroactive steroid levels are modified in cerebrospinal fluid and plasma of post-finasteride patients showing persistent sexual side effects and anxious/depressive symptomatology. J Sex Med 2013;10:2598-603.
[Google Scholar]
|
| 43. |
Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med 2011;8:1747-53.
[Google Scholar]
|
| 44. |
Ganzer CA, Jacobs AR, Iqbal F. Persistent Sexual, Emotional, and Cognitive Impairment Post-Finasteride: A Survey of Men Reporting Symptoms. Am J Mens Health 2014 [In Press].
[Google Scholar]
|
| 45. |
Available from: http:// www.ishrs.org/article/update-international - society-hair-restoration-surgery-task - force - finasteride -adverse-event. [Last accessed on 2014 Sep 08].
[Google Scholar]
|
| 46. |
Holle H, Warne K, Seth AK, Critchley HD, Ward J. Neural basis of contagious itch and why some people are more prone to it. Proc Natl Acad Sci U S A 2012;109:19816-21.
[Google Scholar]
|
| 47. |
Papoiu AD, Wang H, Coghill RC, Chan YH, Yosipovitch G. Contagious itch in humans: A study of visual 'transmission' of itch in atopic dermatitis and healthy subjects. Br J Dermatol 2011;164:1299-303.
[Google Scholar]
|
| 48. |
Gollnick H, Blume-Peytavi U, Szabó EL, Meyer KG, Hauptmann P, Popp G, et al. Systemic isotretinoin in the treatment of rosacea-doxycycline- and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges 2010;8:505-15.
[Google Scholar]
|
| 49. |
Tangrea JA, Adrianza E, Helsel WE, Taylor PR, Hartman AM, Peck GL, et al. Clinical and laboratory adverse effects associated with long-term, low-dose isotretinoin: Incidence and risk factors. The Isotretinoin-Basal Cell Carcinomas Study Group. Cancer Epidemiol Biomarkers Prev 1993;2:375-80.
[Google Scholar]
|
| 50. |
Liccardi G, Senna G, Russo M, Bonadonna P, Crivellaro M, Dama A, et al. Evaluation of the nocebo effect during oral rechallenge in patients with adverse drug reactions. J Investig Allergol Clin Immunol 2004;14:104-7.
[Google Scholar]
|
| 51. |
Lombardi C, Gargioni S, Canonica GW, Passalacqua G. The nocebo effect during oral challenge in subjects with adverse drug reactions. Eur Ann Allergy Clin Immunol 2008;40:138-41.
[Google Scholar]
|
| 52. |
Bavbek S, Aydýn O, Sözener ZC, Yüksel S. Determinants of nocebo effect during oral drug provocation tests. Allergol Immunopathol (Madr) 2014 [In Press].
[Google Scholar]
|
| 53. |
O'Connor AM, Pennie RA, Dales RE. Framing effects on expectations, decisions, and side effects experienced: The case of influenza immunization. J Clin Epidemiol 1996;49:1271-6.
[Google Scholar]
|
| 54. |
Drake L, Hordinsky M, Fiedler V, Swinehart J, Unger WP, Cotterill PC, et al. The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. J Am Acad Dermatol 1999;41:550-4.
[Google Scholar]
|
| 55. |
Sardana K, Garg VK. Efficacy of low-dose isotretinoin in acne vulgaris. Indian J Dermatol Venereol Leprol 2010;76:7-13.
[Google Scholar]
|
| 56. |
Rajput RJ. Cyclical Medicine for hair loss management and improved results in hair transplantation. Hair Transpl Forum Int 2008;18:208-10.
[Google Scholar]
|
Fulltext Views
8,337
PDF downloads
3,961





