Translate this page into:
Non-pharmacological therapies and their efficacy in atopic dermatitis: A narrative review
Corresponding author: Dr. Rahul Mahajan, Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. drrahulpgi@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Hemalatha M, Dev A, Mahajan R, De D, Handa S. Non-pharmacological therapies and their efficacy in atopic dermatitis: A narrative review. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1076_2024
Abstract
Atopic dermatitis (AD) is a complex immune-mediated disease characterised by recurrent eczematous lesions and pruritus, which adversely affects the quality of life (QoL). Genetic factors, environmental factors, immune dysregulation, and skin barrier dysfunction contribute to its pathophysiology. Non-pharmacological management strategies aim to preserve the skin barrier, address immune dysregulation, and minimise triggers. In this review, wediscuss various non-pharmacological interventions, including allergen (aeroallergens, food allergens, and contact allergens) avoidance, bathing-related measures, moisturisers, clothing choices, therapies targeting the skin microbiome, and allergen-specific immunotherapy, in addition to education and psychotherapy. Non-pharmacological therapies are essential for the holistic management of AD, but their effectiveness varies, highlighting the need for further research and tailored approaches to individual patient needs.
Keywords
Allergens
atopic dermatitis
moisturisers
non-pharmacological
Introduction
Atopic dermatitis (AD) is an immune-mediated disease influenced by multiple factors. It involves recurrent episodes of eczematous lesions, secondary skin infections, skin irritation, and pruritus that adversely impact patients’ quality of life (QoL). It affects 15-20% of children and 2-5% of adults globally.1,2 The pathophysiology of AD is intricate and multifaceted, involving components such as skin barrier disruption, dysregulated cell-mediated immune responses, environmental factors, genetics, IgE-mediated hypersensitivity, and microbial imbalance. Non-pharmacological management involves the use of approaches other than drugs as opposed to pharmacological management, which entails treatment using topical and oral medications to control exacerbations and maintain disease remission. Most non-pharmacological interventions focus on preserving the skin barrier, avoiding triggers, addressing immunological dysregulation, correcting skin-gut axis dysbiosis, and minimising bacterial colonisation. However, the efficacy of these measures varies across patients and studies. This review critically examines these approaches and their roles in treating AD. Though the role of non-pharmacological measures in the management of AD is well known, there is limited published literature on the role and efficacy of these methods. This review aims to highlight their efficacy, and limitations which will aid in more comprehensive counselling of patients and parents for control of AD.
Methods
A literature search was conducted in PubMed, EMBASE, and the Cochrane Library using the keywords ‘allergens,” ‘allergy,” ‘atopic dermatitis,” ‘moisturisers,” ‘emollients,” ‘bath/bathing,” ‘cleansing,” ‘clothing,” ‘prebiotics,” ‘probiotics,” ‘symbiotics,” ‘skin microbiome,” ‘immunotherapy,”‘psychotherapy,”‘education,” and ‘non-pharmacological treatments” for articles published between 1964 and 2024. Boolean search strategy was used to combine the keywords in relevant combinations
A total of 110 relevant studies were included, consisting of 35 randomised controlled trials and systematic reviews, 10 epidemiological studies, 15 retrospective studies, 5 guidelines, and 45 review articles. The studies which dealt with clinical outcomes were preferred for inclusion, studies that were entirely experimental, microbial, or immunological were preferably excluded with a few exceptions namely studies that dealt with the role of allergens, water hardness, pH of the skin, and role of the gut microbiome. There were 12 Indian studies, including 4 RCTs, systematic reviews, 4 review articles, and 7 pilot studies.
Aeroallergens
Figure 1 depicts the pathogenesis of AD and the various non-pharmacological strategies targeting each factor.
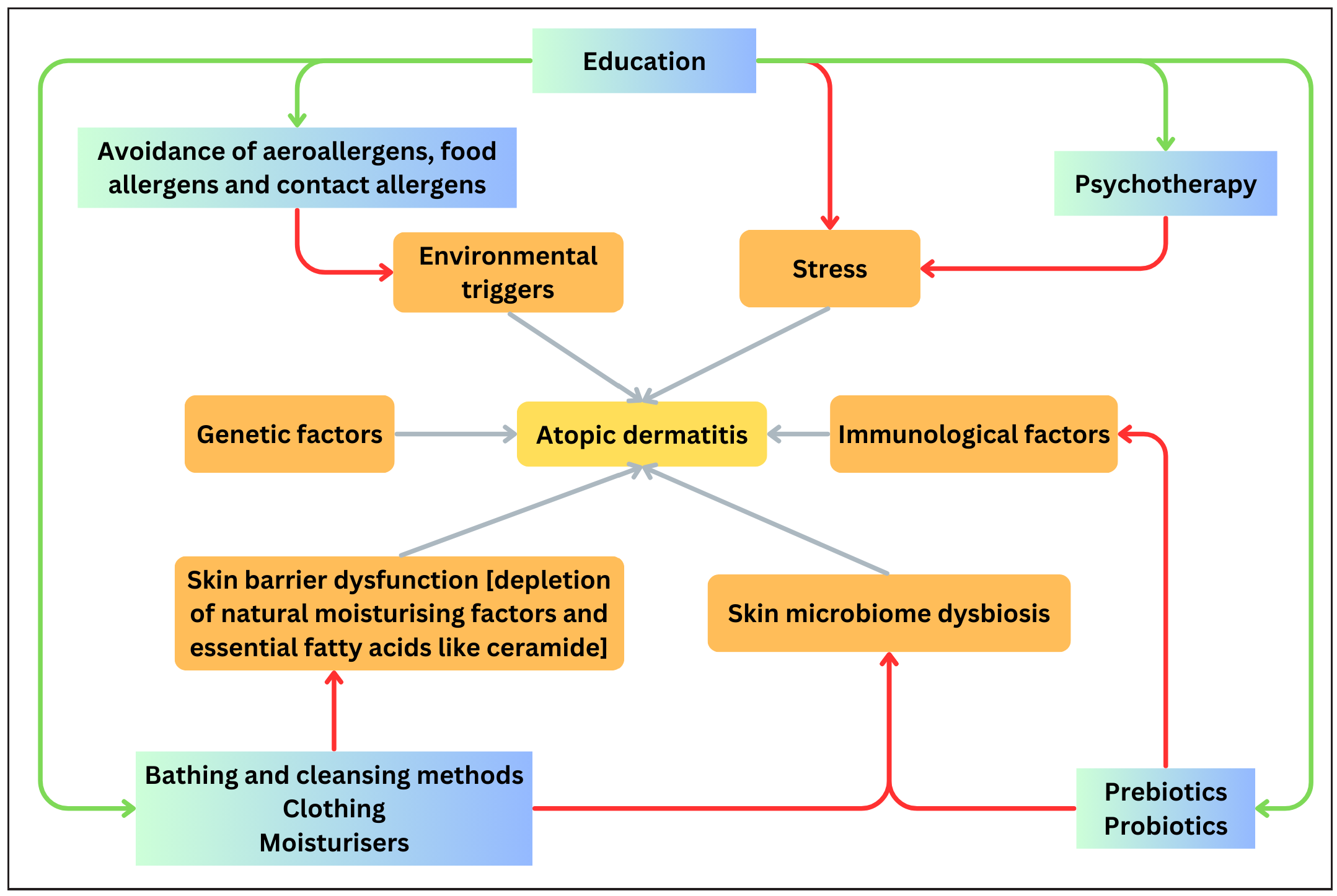
- Flowchart depicting the pathogenetic mechanisms of AD and the various non-pharmacological strategies targeting each factor.
Various allergens, including aeroallergens, food allergens, chemicals, hard water, and textiles like wool, have been known to exacerbate AD .3 Amongst aeroallergens, the most commonly implicated ones are house dust mites (, cat epithelia, and pollen.4 Aeroallergens are implicated when a patient has asthma, allergic rhinoconjunctivitis along with AD, a history of seasonal worsening of the dermatitis in an airborne distribution pattern, and relief in lesions with a change of environment.5-9
1. Role of house dust mite
Dermatophagoides pterynosinnus and Dermatophagoides farinae are the species of house dust mite most frequently linked to the exacerbation of AD. The major clinically relevant allergens are Der p 1, Der p 2, Der p 23, Der p 5, Der p 7, and Der p 21.10 These are proteases that trigger the disruption of tight junctions in the skin and bronchial mucosa.11 Proteases disrupt the epidermal barrier, acting as haptens and IgE antigens on impaired skin. Patients with elevated IgE levels and a history of asthma are more susceptible to atopic dermatitis exacerbations triggered by dust mites.12 The possible methods that can be employed to reduce exposure to aeroallergens include mattress encasings, cleaning with high-efficiency particulate air filter-equipped (HEPA) vacuum cleaners, and using acaricides such as tannic acid, benzyl benzoate, neem oil, and eucalyptus oil.13-15 Other easy methods include clearing the bedroom of fluffy toys, upholstery, curtains, sheepskins, and pillows, as well as frequently washing the bedding, flooring, and drapes.16 Table 1 elaborates on the personal experiences of the authors along with anecdotal evidence in the role of control of aeroallergens and other non-pharmacological methods in AD.
| Target area | Prevention strategies | |
|---|---|---|
| Aeroallergens | Prevention methods for house dust mite removal |
|
| Handling of soft furnishings |
|
|
| Strategies of washing |
|
|
| Role of sun-drying |
|
|
| Role of ventilation and vacuum cleaning |
|
|
| Role of seasonal variation |
|
|
| Role of incense cones or sticks (dhoop or agarbattis) |
|
|
| Role of fabric dyes |
|
|
| Prevention strategies for pollen |
|
|
| Food allergens | Identification and confirmation of food allergens |
|
| Skin microbiome |
|
|
| Role of sodium bicarbonate bath |
|
|
| Skin barrier maintenance | Role of oils |
|
| Complementary and alternative medicine | Acupuncture, acupressure, hypnotherapy, relaxation and massage, balneotherapy, herbal therapies, oral oils and fatty acids |
|
| Allergen-specific immunotherapy (AIT) | Role of AIT along with oral immunosuppressants |
|
| Holistic management |
|
|
HDM: House dust mite, TEWL: Trans epidermal water loss
Der p 1 and Der p 1 plus Der f 1 allergen concentrations significantly decreased in a study using house dust mite -impermeable encasings, but neither the non-house dust mite mattress encasing group nor the house dust mite -mattress encasing group experienced significant changes in clinical parameters as a result of this decrease in allergen load.17 Two studies have demonstrated a definite advantage of house dust mite avoidance measures in the amelioration of AD.18,19 Seven randomised controlled studies were included in a Cochrane review to assess the efficacy of various house dust mite prevention techniques. It had a high risk of bias as there was heterogeneity in the interventions performed as well as outcome measures. Of the three studies that evaluated the reduction in severity score after implementing multiple avoidance strategies, only one study showed a statistically significant effect, while the other two did not.13 Based on the currently available evidence, we cannot conclude the benefit of various house dust mite reduction strategies in clinical scenarios. Although frequent cleaning with a high-efficiency particulate air filter-equipped filter-equipped vacuum cleaner reduces mite load and allergens, no studies compared the clinical improvement with this strategy.20,21
2. Role of pollen allergy
Studies have shown that exposure to grass pollen intensifies itching and severity of AD.22,23 Pollen-induced allergy is suspected when symptoms deteriorate during pollen seasons, like summer and spring, and improve with reduced exposure.
3. Role of pet allergens
A pet allergy is suspected when a patient’s symptoms aggravate following contact with pets, and the patient has rhinoconjunctivitis and/or asthma.9,24 The uteroglobin-like protein Fel d 1 antigen is the most common and well-studied cat allergen.24 Allergic sensitisation can be detected using serum-specific IgE or skin prick testing (SPT) with allergen extracts.25 If definite evidence of pet allergy is present, contact with animals must be avoided. Although cats are the most usually implicated in pet allergies, it is best to avoid dogs as well to avoid skin infections in patients with AD.3,26
Pollutants
Indoor and outdoor pollutants, including tobacco smoke and traffic exhaust, have also been reported to contribute to the development, progression, and persistence of AD.3 A meta-analysis of observational studies indicates that both active and passive smoking exposure increases the prevalence of AD in children and adults, with an inconsistent association with maternal smoking.27
Food allergens
Food allergy is suspected in cases of severe AD that quickly worsen when medication is ceased, in cases with a reliable history of immediate allergic reactions to specific foods, and in children under 5 years with recalcitrant AD.28 An IgE-specific food allergy is present in 40% of newborns and young children with moderate to severe AD and 8% of children overall. Cow’s milk, hen’s egg, peanut, soya, almonds, seafood, nightshades, food-colouring agents, food additives, and preservatives are the most common food allergens responsible for AD aggravation in young children.28 Older children, adolescents, and adults may experience pollen-related food allergies due to cross-reactivity with plant proteins. Symptoms typically involve the oropharyngeal mucosa and occur within 5-10 minutes after eating certain fresh fruits, vegetables, nuts, legumes, and seeds.29-31
Maintaining a food diary can help identify potential food triggers for AD flares, particularly in children with suspected food allergies. We can perform in-vivo tests skin prick test (SPT), in-vitro tests (serum-specific IgE), and atopic patch testing (APT) when a patient is suspected to have a food allergy. SPT is excellent for detecting immediate anaphylactic IgE-mediated reactions. Wheal diameters ≥8mm for milk and peanut and ≥7mm for egg are used as cut-offs since they have 100% positive predictive value.32 Atopic patch testing detects non-anaphylactic delayed reactions, but it is not standardised for commonly consumed foods and is performed using fresh food items diluted in saline.3,33 Skin prick test has a lower sensitivity and can be challenging for young children due to limited cooperation, fear of needles, and heightened anxiety.33 Atopic patch testing has limitations like lack of standardisation, skin irritation, and angry back syndrome; it is also time-consuming.34
In vitro tests are effective when skin prick test cannot be employed (e.g. dermographism or UV- and drug-induced skin hyporeactivity, dermatitis at the test site, or lack of cooperation in young children).3 They provide quantitative data on the degree of sensitisation, which aids in estimating the likelihood of a clinical reaction. An IgE cut-off ≥0.35 kUA/L is widely accepted to determine a test as positive, but it should be clinically correlated.35 However, the gold standard for detection of food allergy is a double-blind placebo-controlled food challenge.36 It should be conducted after the patient has abstained from the suspected food for four to six weeks. Avoiding the trigger food item lessens disease severity in patients with a confirmed food allergy. Current research suggests that for children with known food allergies and documented true IgE-mediated allergies, it is advisable to avoid only the foods they reacted to rather than broadly eliminating many items.37 Recent studies like the Enquiring About Tolerance (EAT) study, Learning Early About Peanut Allergy (LEAP) study, and Preventing Atopic dermatitis and ALLergies in Children (PreventADALL)studies indicate that introducing a variety of complementary foods to high-risk infants at 4-6 months of age can help in averting the occurrence of food allergies.38-40
Contact allergens
Patients with moderate-to-severe AD that is resistant to treatment and has an atypical pattern or distribution affecting the hands, feet, lips, and eyelids with an onset in adolescence or adulthood should be evaluated for contact allergy. Nickel, perfumes, rubber compounds, formaldehyde, neomycin, and lanolin are the commonly implicated allergens. According to an Indian study, patients with severe AD demonstrated positive patch test results with fragrance mix, nickel, cobalt chloride hexahydrate, and potassium dichromate, which were the most commonly detected allergens, with the fragrance mix being the most prevalent allergen.41
Bathing and cleansing
Skin cleansing is essential; however, it must be done gently to preserve the epidermal barrier. Mechanical cleansing is more essential. It is recommended to take a 5 to 10-minute bath in lukewarm water (27 to 30°C).3,16 A systematic review concluded that daily baths or showers did not correlate with the exacerbation of AD and therefore, should be encouraged in AD patients.17 Commercially available soaps include prototype anionic surfactants, glycerine bars, superfatted soaps, antibacterial soaps, syndets/cleansing bars, and novel cleansers that contain emollients. Soaps consist of anionic surfactants with a pH of 9 to 10, whereas bar and liquid syndets have a slightly acidic or neutral pH.42-43 The alkalinity of commonly available cleansing products disturbs the skin’s acid mantle layer, leading to increased permeability, decreased antimicrobial activity, and modified enzymatic activity.44,45 Hence, cleansing agents with a neutral pH are preferred.46
Antiseptic agents that can be added to the bath are bleach and potassium permanganate (KMnO4).
1. Bleach bath:
The ideal bleach concentration is 2.5 µL/mL, or 0.005% NaOCl, which can be achieved by dissolving half a cup of 6% normal household bleach in a standard 150 L bathtub of water or one tablespoon for 20 litres bucket of water.47 Two systematic reviews demonstrate that bleach baths improve AD severity scores, but there is no conclusive evidence for their superiority over water baths.48,49 However, it is not well accepted by Indian patients, though it is appropriate for resource-poor situations.50 Modifications like bleach suits can also be employed, which involves soaking a cotton pyjama suit in a dilute bleach solution and having the child wear it for 10 minutes, 2–3 times a week.51
2. KMnO4 bath:
The optimal dilution of KMnO4 for medicinal usage is 1:10,000, which can be obtained by mixing 400 mg KMnO4 in 4L of water.52 For ease, dilute the solution to reach a pink colour that matches with nail bed is achieved. KMnO4 possesses an oxidising property that aids in the prevention of secondary infections, as well as anti-pruritic and anti-inflammatory qualities that aid the drying up of denuded areas.53 Though the use and benefits of KMnO4 are known, evidence in the literature is limited.54
Role of water hardness:
Hard water may contribute to epidermal barrier defects.43 One study found no correlation between hardness of water and an increased risk of AD,55 despite high levels of calcium carbonate (hardness of water) being positively associated with an increased risk of AD in younger children.56-59 On the other hand, in a systematic review of 16 studies, 7 showed a relationship between higher CaCO3 levels and increased development of AD in children.60 However, no discernible positive effect has been observed with the use of water softeners.56-59,61,62
Moisturisers
Moisturisers are a class of products that increase skin hydration by increasing water retention in the stratum corneum (humectant), preventing water loss (occlusive), and smoothing the skin’s surface by forming a protective coating (emollient).63 They play a prime role in the management of AD by restoring epidermal barrier function. Emollients commonly include fatty acids, fatty alcohols, cholesterol, squalene, natural plant oils, ceramides, and pseudo-ceramides. Humectants include urea, sorbitol, panthenol, glycerol, propylene glycol, hyaluronic acid, and α-hydroxy acids. Mineral oil, petroleum jelly, beeswax, silicones, zinc oxide, shea/ mango/cocoa butter, and paraffin act as occlusives. Protein rejuvenators are a newer class of moisturisers that include collagen, elastin, and keratin. Natural oils like coconut oil, olive oil, sunflower oil, safflower oil, mustard oil, sweet almond oil, jojoba oil, and evening primrose oil are widely utilised. The fatty acid content in the oil determines its effects. Large amounts of linoleic acid support barrier function by activating Peroxisome proliferator-activated receptor alpha (PPAR-α), promoting keratinocyte growth, lipid synthesis, and modulating inflammation.64,65 In contrast, oleic acid impairs barrier function by increasing transepidermal water loss, permeability, and inflammation, and has limited antibacterial properties.66,67 Coconut, sunflower, and safflower oils are preferred due to their higher levels of beneficial fatty acids, while olive oil is not recommended due to its high oleic acid levels.64,68
Two systematic reviews reported that moisturisers extended the flare-free period, reduced topical corticosteroid use, and alleviated pruritus and xerosis, but did not affect objective scores69,70 It is difficult to choose an appropriate moisturiser due to the heterogeneity of the individual components in commercial formulations. There is a dearth of evidence on the relative efficacies and superiority among various moisturisers. However, ceramide-based moisturisers have been meticulously investigated.71-74 A systematic review which compared the efficacy of various moisturisers with ceramide-based formulations revealed that ceramide-based moisturisers were superior in terms of improvement in SCORAD.75
Although there is a suggestion that emollient therapy starting at birth is a practical, safe, and successful strategy for preventing the future development of AD, larger studies such as the Preventing Atopic dermatitis and ALLergies in Children (PreventADALL) and Barrier Enhancement for Eczema Prevention (BEEP) trials failed to find any advantage in using emollients early in life.39,76 Emollients therefore help in maintaining remission, but not in disease prevention.
Wet wrap therapy
Wet-wrap therapy involves applying wet bandages over areas of atopic eczema after using emollients or topical corticosteroids to enhance skin hydration, reduce pruritus, and improve medication penetration. Though it increases the risk of skin infections,77 this risk is not significant in short-term paediatric use and is outweighed by its efficacy in refractory and acute cases.
Clothing
Conventionally, people with AD are advised to avoid wool and to wear cotton and silk garments, because of the softness, breathability, and comfort of the latter.78,79 Nevertheless, even cotton short interwoven fibres and damp fabrics irritate the skin, while superfine or ultrafine merino wool is well tolerated.80 Promising novel materials are being developed for AD patients, including cellulose-based, chitosan-coated, and silver-coated materials, which help decrease the severity of disease or the S. aureus burden.81 In a study, patients of AD preferred lyocell, a synthetic cellulose fibre, over cotton, but there are limited data and varied results with other materials, making it difficult to choose the ideal apparel.82
Skin and gut microbiome
Disruption of the skin microbiome in patients with AD results in decreased normal flora and colonisation by Staphylococcus aureus. Through the stimulation of various T-cell clones and cytokines (IL-3, histamine, and IL-13), virulence factors secreted by S. aureus colonies, including superantigens, α-toxin, δ-toxin, protein A, and phenol-soluble modulins (PSM), exacerbate inflammation and enhance the inflammatory response.83-85 Topical probiotics containing organisms such as Lactobacillus johnsonii, Streptococcus thermophilus, and lysates of Vitreoscilla filiformis have been used in creams to help restore microbial balance and improve skin microbiome.
Gut microbes play a crucial role in regulating systemic inflammation, and an imbalanced gut microbiome, and less diverse gut microbiome has been linked to the development of AD. Correction of gut dysbiosis has been undertaken by multiple authors in the form of probiotics, prebiotics, postbiotics, and synbiotics.
Probiotics
Probiotics are live, beneficial microbes that have an immunomodulatory effect by lowering proinflammatory cytokines (IL-4, IL-6, tumour necrosis factor-α, and INF-γ), suppressing Th2 response, and increasing the Th1/Th2 ratio.86,87 They include non-pathogenic yeast like Saccharomyces boulardii and bacteria from Bifidobacterium, Streptococcus, and Lactobacillus families.88 The dosage of Lactobacillus ranges from 5×109 to 1x1010 CFU, administered once or twice daily for 4 to 12 weeks.89 The dosage of Bifidobacterium ranges from 3×109 to 1×1010 CFU, given once or twice daily.89 Probiotics have been shown to have beneficial effects in systematic reviews; however, 2 studies failed to show beneficial effects.90-93
Prebiotics
Prebiotics are nondigestible compounds (fructo-oligosaccharides, galacto-oligosaccharides, and long-chain inulin) that promote the growth of beneficial micro-organisms.94,95 They increase the formation of short-chain fatty acids (acetate, propionate, and butyrate), which have anti-inflammatory properties and enhance the Th1/Th2 ratio.96,97
Postbiotics
Postbiotics are composed of inanimate microbes and/or their components that offer immunomodulatory, anti-inflammatory, and antimicrobial benefits without the ability to colonise the host.98 Postbiotics offer several advantages, including a well-defined chemical composition, lack of antibiotic resistance transfer, greater stability, and extended shelf life.99 A systematic review reported symptom improvement with oral Lactobacillus postbiotics for AD in adults, but findings were inconsistent in paediatric patients, due to varying dosages.100
Synbiotics
A synbiotic has a mixture of prebiotics and probiotics, thus utilising the synergistic effects of these components. While some studies suggest that synbiotics may enhance treatment outcomes, their superiority over prebiotics or probiotics alone remains debatable.101 A recent study found no significant difference in SCORAD scores between patients treated with synbiotics versus those receiving prebiotics alone.102
Educational intervention
This involves providing sufficient time to explain the condition, discussing the importance of compliance with proper skin care, implementing behavioural modifications through patient and parental education, and addressing patient concerns.103 It can be facilitated by dermatologists, paediatricians, psychiatrists, psychologists, and nurses.104 Group discussions with parents of similar-age children are also essential to address shared concerns, reduce isolation, and provide support for families with AD, fostering a sense of community and validation.103
Psychotherapy
Psychological and emotional aspects are recognised factors that modify the clinical course of AD.105 Stress can aggravate the disease and alter the itch-scratch cycle.106 Itching in AD can impair sleep quality, leading to mental health issues such as depression, anxiety, and there can be social isolation due to visible skin lesions. A meta-analysis found the odds of depression, anxiety, sleep disorders, and conduct disorder to be 1.42, 1.33, 2.10, and 1.49, respectively.107 Psychoeducation and psychotherapeutic interventions like cognitive behavioural therapy and interpersonal therapy can help in improving the QoL of patients.
Allergen-specific immunotherapy (AIT)
Patients with respiratory allergies and stinging insect hypersensitivity may benefit from allergen-specific immunotherapy.108 This involves administering increasing dosages of the allergen gradually to modify the response and encourage peripheral immunological tolerance mechanisms, which leads to a shift from a Th2 response to a Th1 response, a decrease in mediator release from mast cells, and the generation of blocking antibodies IgG4.106 This desensitisation is performed via sublingual or subcutaneous routes. In one systematic review with eight randomised controlled trials, there was a statistically significant benefit in patients receiving allergen-specific immunotherapy when compared to placebo, but there was a high level of heterogeneity.109 In another systematic review with 23 RCTs, immunotherapy to house dust mites demonstrated improvement in AD severity, but the impact on long-term AD control and flares was less certain.110
Conclusion
Non-pharmacological therapies have an important role in maintaining the skin barrier function, preventing exacerbation due to allergen exposure, preventing skin infections, preserving the skin-gut microbiome, and overall improving the overall QoL of patients with AD. Evidence in the literature is heterogeneous, and clinical experience varies across different settings, especially for these therapies as standalone modalities. Nevertheless, dermatologists and physicians need to be aware of the various components of non-pharmacological therapies in order to provide holistic care for patients with AD.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733-43.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy. 2018;73:1284-93.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus-based european guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J EurAcad Dermatol Venereol. 2018;32:657-82.
- [Google Scholar]
- Prevalence of common sensitizing aeroallergens in patients with atopic dermatitis. Cytokine. 2023;162:156087.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic endotypes and phenotypes of asthma. J Allergy Clin Immunol Pract. 2020;8:429-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Common aeroallergens in patients with asthma and allergic rhinitis living in southwestern part of Iran: Based on skin prick test reactivity. Iran J Allergy Asthma Immunol. 2015;14:133-8.
- [PubMed] [Google Scholar]
- Aeroallergen sensitization in asthma: Prevalence and correlation with severity. Allergy Asthma Proc. 2010;31:96-102.
- [CrossRef] [PubMed] [Google Scholar]
- Component-Resolved diagnosis in allergic rhinitis and asthma. J Appl Lab Med. 2019;3:883-98.
- [CrossRef] [PubMed] [Google Scholar]
- Nonpharmacological management of atopic dermatitis. Indian Journal of Paediatric Dermatology. 2017;18:166-73.
- [CrossRef] [Google Scholar]
- House dust mite allergy: The importance of house dust mite allergens for diagnosis and immunotherapy. Mol Immunol. 2023;158:54-67.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatophagoidespteronyssinus major allergen 1 activates the innate immune response of the fruit fly drosophila melanogaster. J Immunol. 2013;190:366-71.
- [CrossRef] [PubMed] [Google Scholar]
- The role of house dust mites and other aeroallergens in atopic dermatitis. Clinics in dermatology. 2003;21:177-82.
- [CrossRef] [PubMed] [Google Scholar]
- House dust mite reduction and avoidance measures for treating eczema. Cochrane Database Syst Rev. 2015;1:CD008426.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Home environmental interventions for house dust mite. J Allergy Clin Immunol Pract. 2018;6:1-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Update on house dust mite allergen avoidance measures for asthma. Curr Allergy Asthma Rep. 2020;20:50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of mattress encasings on atopic dermatitis outcome measures in a double-blind, placebo-controlled study: The dutch mite avoidance study. J Allergy Clin Immunol. 2002;110:500-6.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of house dust mite avoidance measures in children with atopic dermatitis. Br J Dermatol. 2000;143:379-84.
- [CrossRef] [PubMed] [Google Scholar]
- Double-blind controlled trial of effect of housedust-mite allergen avoidance on atopic dermatitis. Lancet. 1996;347:15-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of physical interventions on house dust mite allergen levels in carpet, bed, and upholstery dust in low-income, urban homes. Environ Health Perspect. 2001;109:815-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of HEPA vacuum cleaning and dry steam cleaning in reducing levels of polycyclic aromatic hydrocarbons and house dust mite allergens in carpets. J Environ Monit. 2009;11:205-11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Seasonality in symptom severity influenced by temperature or grass pollen: Results of a panel study in children with eczema. J Invest Dermatol. 2005;124:514-23.
- [CrossRef] [PubMed] [Google Scholar]
- Exacerbation of atopic dermatitis on grass pollen exposure in an environmental challenge chamber. J Allergy Clin Immunol. 2015;136:96-103.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular diagnosis in cat allergy. World J Methodol. 2021;11:46-60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dog and cat allergies: Current state of diagnostic approaches and challenges. Allergy Asthma Immunol Res. 2018;10:97-105.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Atopic dermatitis and the hygiene hypothesis revisited. Curr Probl Dermatol. 2011;41:1-34.
- [CrossRef] [PubMed] [Google Scholar]
- Association of atopic dermatitis with smoking: A systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:1119-25.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An uncontrolled open pilot study to assess the role of dietary eliminations in reducing the severity of atopic dermatitis in infants and children. Indian J Dermatol. 2009;54:183-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Position paper of the EAACI: Food allergy due to immunological cross-reactions with common inhalant allergens. Allergy. 2015;70:1079-90.
- [CrossRef] [PubMed] [Google Scholar]
- Oral allergy syndrome: A clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010;104:101-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pollen-Food allergy syndrome: A not so rare disease in childhood. Medicina (Kaunas). 2019;55:641.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The diagnostic value of skin prick testing in children with food allergy. Pediatr Allergy Immunol. 2004;15:435-41.
- [CrossRef] [PubMed] [Google Scholar]
- Skin prick test in patients with chronic allergic skin disorders. Indian J Dermatol. 2015;60:159-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Atopic patch testing. Indian J Dermatol Venereol Leprol. 2008;74:467-70.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers of diagnosis and resolution of food allergy. Pediatr Allergy Immunol. 2021;32:223-33.
- [CrossRef] [PubMed] [Google Scholar]
- EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy. 2014;69:1008-25.
- [CrossRef] [PubMed] [Google Scholar]
- The role of elimination diets in atopic dermatitis-A comprehensive review. Pediatr Dermatol. 2017;34:516-27.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-13.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Early food intervention and skin emollients to prevent food allergy in young children (PreventADALL): A factorial, multicentre, cluster-randomised trial. Lancet. 2022;399:2398-11.
- [CrossRef] [PubMed] [Google Scholar]
- Enquiring about tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol. 2016;137:1477-86.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Contact hypersensitivity to indian standard patch test series correlates with disease severity among children with atopic dermatitis. Indian J Dermatol Venereol Leprol. 2023;90:46-51.
- [CrossRef] [PubMed] [Google Scholar]
- Cleansers and their role in various dermatological disorders. Indian J Dermatol. 2011;56:2-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bathing and associated treatments in atopic dermatitis. Am J Clin Dermatol. 2017;18:45-57.
- [CrossRef] [PubMed] [Google Scholar]
- The acid mantle: A myth or an essential part of skin health? Curr Probl Dermatol. 2018;54:1-10.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of acidification in atopic eczema: An underexplored avenue for treatment. J Clin Med. 2015;4:970-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The pH of the skin surface and its impact on the barrier function. Skin PharmacolPhysiol. 2006;19:296-302.
- [CrossRef] [Google Scholar]
- Efficacy of bleach baths in reducing severity of atopic dermatitis: A systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2017;119:435-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bleach baths for atopic dermatitis: A systematic review and meta-analysis including unpublished data, bayesian interpretation, and GRADE. Ann Allergy Asthma Immunol. 2022;128:660-8.
- [CrossRef] [PubMed] [Google Scholar]
- Use of bleach baths for atopic dermatitis: An indian perspective. Indian J Dermatol. 2022;67:273-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bleach suit for atopic dermatitis. Pediatr Dermatol. 2024;41:726-7.
- [CrossRef] [PubMed] [Google Scholar]
- Topical potassium permanganate solution use in dermatology: Comparison of guidelines and clinical practice. Clin Exp Dermatol. 2022;47:966-7.
- [CrossRef] [PubMed] [Google Scholar]
- What is the evidence for the use of potassium permanganate for wound care? Drug Ther Bull. 2020;58:71-4.
- [CrossRef] [PubMed] [Google Scholar]
- Drugs for eczema of children. Can Med Assoc J. 1964;90:693-4.
- [PubMed] [PubMed Central] [Google Scholar]
- Water hardness and eczema at 1 and 4 y of age in the INMA birth cohort. Environ Res. 2015;142:579-85.
- [CrossRef] [PubMed] [Google Scholar]
- Interactions between domestic water hardness, infant swimming and atopy in the development of childhood eczema. Environ Res. 2012;116:52-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ecological association of water hardness with prevalence of childhood atopic dermatitis in a japanese urban area. Environ Res. 2004;94:33-7.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic eczema and domestic water hardness. Lancet. 1998;352:527-31.
- [CrossRef] [PubMed] [Google Scholar]
- Association between domestic water hardness, chlorine, and atopic dermatitis risk in early life: A population-based cross-sectional study. J Allergy Clin Immunol. 2016;138:509-16.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of water hardness on atopic eczema, skin barrier function: A systematic review, meta-analysis. Clin Exp Allergy. 2021;51:430.
- [CrossRef] [PubMed] [Google Scholar]
- A multicentre randomised controlled trial and economic evaluation of ion-exchange water softeners for the treatment of eczema in children: The softened water eczema trial (SWET) Health Technol Assess. 2011;15:v-vi.
- [CrossRef] [PubMed] [Google Scholar]
- A randomised controlled trial of ion-exchange water softeners for the treatment of eczema in children. PLoS Med. 2011;8:e1000395.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The role of moisturizers in addressing various kinds of dermatitis: A review. Clin Med Res. 2017;15:75-87.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of olive and sunflower seed oil on the adult skin barrier: Implications for neonatal skin care. Pediatr Dermatol. 2013;30:42-50.
- [CrossRef] [PubMed] [Google Scholar]
- The benefits of sunflower oleodistillate (SOD) in pediatric dermatology. Pediatr Dermatol. 2009;26:669-75.
- [CrossRef] [PubMed] [Google Scholar]
- Function of oleic acid on epidermal barrier and calcium influx into keratinocytes is associated with n-methyl d-aspartate-type glutamate receptors. Br J Dermatol. 2009;160:69-74.
- [CrossRef] [PubMed] [Google Scholar]
- Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972;2:23-8.
- [CrossRef] [PubMed] [Google Scholar]
- Use of ‘natural” oils for moisturization: Review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of moisturizers in paediatric atopic dermatitis: A systematic review and meta-analysis of randomised controlled trials. Indian J Dermatol Venereol Leprol. 2021;88:22-31.
- [CrossRef] [PubMed] [Google Scholar]
- Emollients and moisturisers for eczema. Cochrane Database Syst Rev. 2017;2:CD012119.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: Changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002;47:198-208.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of a paraffin-based moisturizer compared to a ceramide-based moisturizer in children with atopic dermatitis: A double-blind, randomized controlled trial. Pediatr Dermatol. 2023;40:627-32.
- [CrossRef] [PubMed] [Google Scholar]
- A daily regimen of a ceramide-dominant moisturizing cream and cleanser restores the skin permeability barrier in adults with moderate eczema: A randomized trial. Dermatol Ther. 2021;34:e14970.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A clinical evaluation of the comparable efficacy of hyaluronic acid-based foam and ceramide-containing emulsion cream in the treatment of mild-to-moderate atopic dermatitis. J Cosmet Dermatol. 2011;10:185-8.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy of moisturisers containing ceramide compared with other moisturisers in the management of atopic dermatitis: A systematic literature review and meta-Analysis. Indian J Dermatol. 2023;68:53-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Emollients for prevention of atopic dermatitis: 5-year findings from the BEEP randomized trial. Allergy. 2023;78:995-1006.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and problems associated with using a wet-wrap garment for children with severe atopic dermatitis. J Dermatolog Treat. 2007;18:301-5.
- [CrossRef] [PubMed] [Google Scholar]
- The role of textiles in dermatitis: An update. Curr Allergy Asthma Rep. 2015;15:17.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical effectiveness of a silk fabric in the treatment of atopic dermatitis. Br J Dermatol. 2004;150:127-31.
- [CrossRef] [PubMed] [Google Scholar]
- Fabric selection in atopic dermatitis: An evidence-Based review. Am J Clin Dermatol. 2020;21:467-82.
- [CrossRef] [PubMed] [Google Scholar]
- Fabric preferences of atopic dermatitis patients. Dermatitis. 2009;20:29-33.
- [CrossRef] [PubMed] [Google Scholar]
- The role of the skin microbiome in atopic dermatitis. Curr Allergy Asthma Rep. 2015;15:65.
- [CrossRef] [PubMed] [Google Scholar]
- Staphylococcus aureus in atopic dermatitis: Strain-specific cell wall proteins and skin immunity. Allergol Int. 2019;68:309-15.
- [CrossRef] [PubMed] [Google Scholar]
- Role of the microbiota in skin immunity and atopic dermatitis. Allergol Int. 2017;66:539-44.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on fecal microbiota. Allergol Int. 2014;63:575-85.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of probiotics supplementation on metabolic health in pregnant women: An evidence based meta-analysis. PLoS One. 2018;13:e0197771.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prebiotics and probiotics in atopic dermatitis. Exp Ther Med. 2019;18:926-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Probiotics for the treatment of atopic dermatitis in children: A systematic review and meta-Analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Treatment efficacy of probiotics on atopic dermatitis, zooming in on infants: A systematic review and meta-analysis. Int J Dermatol. 2018;57:635-41.
- [CrossRef] [PubMed] [Google Scholar]
- The role of probiotics in the treatment of adult atopic dermatitis: A meta-analysis of randomized controlled trials. J Health Popul Nutr. 2022;41:37.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of probiotic lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol. 2003;111:389-95.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective, randomized controlled trial on lactobacillus rhamnosus in infants with moderate to severe atopic dermatitis. Br J Dermatol. 2006;155:1256-61.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr Res Rev. 2004;17:259-75.
- [CrossRef] [PubMed] [Google Scholar]
- Probiotics and prebiotics in the prevention and treatment of atopic dermatitis. Pediatr Allergy Immunol Pulmonol. 2016;29:174-80.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The immune-enhancing effects of dietary fibres and prebiotics. Br J Nutr. 2002;87 Suppl2:S221-30.
- [CrossRef] [PubMed] [Google Scholar]
- The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18:649-67.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prebiotics, probiotics, synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules. 2021;11:1903.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Oral postbiotics as a therapeutic strategy for atopic dermatitis: A systematic review of randomized controlled trials. J Am Nutr Assoc. 2024;43:139-46.
- [CrossRef] [PubMed] [Google Scholar]
- World gastroenterology organisation global guidelines: Probiotics and prebiotics october 2011. J Clin Gastroenterol. 2012;46:468-81.
- [CrossRef] [PubMed] [Google Scholar]
- Prebiotics and synbiotics: Two promising approaches for the treatment of atopic dermatitis in children above 2 years. Allergy. 2006;61:431-7.
- [CrossRef] [PubMed] [Google Scholar]
- Educational programs for the management of childhood atopic dermatitis: An integrative review. Asian Nurs Res (Korean Soc Nurs Sci). 2015;9:185-93.
- [CrossRef] [PubMed] [Google Scholar]
- Pediatric atopic eczema: The impact of an educational intervention. Pediatr Dermatol. 2006;23:428-36.
- [CrossRef] [PubMed] [Google Scholar]
- Psychodermatological aspects of atopic dermatitis. Br J Dermatol. 2014;170 Suppl 1:38-43.
- [CrossRef] [PubMed] [Google Scholar]
- European guideline (EuroGuiDerm) on atopic eczema - part II: Non-systemic treatments and treatment recommendations for special AE patient populations. J EurAcad Dermatol Venereol. 2022;36:1904-26.
- [Google Scholar]
- Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: A systematic review and meta-analysis. J Am Acad Dermatol. 2018;79:448-56.
- [CrossRef] [PubMed] [Google Scholar]
- Allergen-specific immunotherapy. Allergy Asthma Clin Immunol. 2018;14:53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy of allergen-specific immunotherapy for atopic dermatitis: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;132:110-7.
- [CrossRef] [PubMed] [Google Scholar]
- Allergen immunotherapy for atopic dermatitis: Systematic review and meta-analysis of benefits and harms. J Allergy Clin Immunol. 2023;151:147-58.
- [CrossRef] [PubMed] [Google Scholar]






