Translate this page into:
Novel drug delivery strategies and gene therapy regimen as a promising perspective for management of psoriasis
Corresponding author: Prof. Sujata Pralhad Sawarkar, SVKM’s Dr. Bhanuben Nanavati College of Pharmacy, Gate No. 1, Mithibai College Campus, V.M. Road, Navpada, Suvarna Nagar, Vile Parle West, Mumbai - 400056, Maharashtra, India. sujata.sawarkar@bncp.ac.in/ sujatasawarkar19@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sawarkar SP, Yadav V. Novel drug delivery strategies and gene therapy regimen as a promising perspective for management of psoriasis. Indian J Dermatol Venereol Leprol 2021;87:333-40.
Abstract
Psoriasis is an autoimmune disorder; however, an exact underlying mechanism responsible for psoriasis is yet not known. A hypothesis put forward is an abnormal proliferation of keratinocytes due to faulty signals brought about by T-cells. Due to the lack of evidence of the exact cause, a variety of treatments have been used of which topical therapy is usually the first option in most patients. Topical therapy has several shortcomings and barriers of drug delivary which may be effectively overcome using novel drug carrier systems which exhibit maximum penetration, controlled release, reduced irritancy and, overall, a better efficacy. Thus, novel treatment strategies based on gene therapy such as antisensing nucleotide, silencing RNA complex, stem cell therapy and antibody-based therapy are being envisaged. This review article discusses the concepts and background of current novel delivery systems and gene therapy tools for effective management of psoriasis.
Keywords
Antibodies
hydrogel
immunomodulatory
nanoparticles
pathogenesis
plaque
psoriasis
psoriasis area severity index
small interfering RNA
steroids
therapy
topical
Introduction
The term psoriasis is derived from Greek work “psora” means “to itch” and “iasis” means “action, condition.”1 Psoriasis is a chronic, noncommunicable, painful, disfiguring and disabling disease for which there is no cure and has a negative impact on the patient’s overall quality of life. Psoriasis affects nearly 2%–3% of the world’s population and presents as erythematous, indurated, scaly plaques on the skin sometimes with involvement of nails and joints.2 The disease can occur at any age.3
Psoriasis is an inflammatory skin disease in which skin cells replicate at an extremely rapid rate. This causes cells to build up on the skin’s surface, forming thick erythematous plaques, covered with flaky, silvery-white scales.
Although psoriasis is usually benign, it progresses to become life long illness with remissions and exacerbations and is sometimes refractory to treatment. Nonetheless, a majority of the cases found in patients are of mild or moderate psoriasis.4 A recent study observed 75.8%of patients had a psoriasis area severity index of <20. Moreover, 17%–55% of patients experienced remissions of varying lengths.5
Plaque-type psoriasis is the most common form, affecting 80%–90% of patients. Inverse, erythrodermic, pustular and guttate forms have also been described as some of the forms of psoriasis.The natural history is variable but is often chronic and relapsing and patients may experience extracutaneous manifestations commonly including nail involvement and psoriatic arthritis in up to 20% of patients.6
Due to altered characteristic of skin due to diseased condition, i.e., psoriasis and side-effects associated with systemic oral and biological therapy, there is an urgent need to look out for newer topical approaches for the management of psoriasis as they overcome the systemic side effects and are also a comparatively cheaper alternative with promising efficacy. However, there are a few drawbacks of conventional topical therapy and, therefore, a need for design and development of novel approaches for effective management of psoriasis.
Pathogenesis of Psoriasis
Exposure to microbial or mechanical injury damage-associated molecular patterns/pathogen-associated molecular patterns leads to activation of antigen-presenting cells like macrophages and dermal dendritic cells; failure to maintain skin barrier due to late cornified envelope proteins 3C/3B deletion leads to continuous exposure to such antigens. Interaction of antigen presenting cells and T cells leads to activation of Th1 and Th17 cells mediated by interleukin-23. Liberation of interleukin-17 and interleukin-22 by Th17 cells, and tumor necrosis factor-α and interferon-γ by Th1 cells further perpetuate the keratinocyte injury creating a vicious positive feedback cycle.7
There are two main hypotheses about the process that occurs in the development of the disease. The first considers psoriasis as primarily a disorder of excessive growth and reproduction of skin cells. The problem is simply seen as a fault of the epidermis and its keratinocytes.
The second hypothesis sees the disease as being an immune-mediated disorder in which the excessive reproduction of skin cells is secondary to factors produced by the immune system. T cells become active, migrate to the dermis and trigger the release of cytokines (tumor necrosis factor-alpha, in particular) which cause inflammation and the rapid production of skin cells.
Current Armamentarium
Wide range of therapeutic approaches have been routinely employed in clinical practice. Various approaches such as topical gels and creams, phototherapy, systemic medications and biologics are used in the treatment of moderate-to-severe psoriasis. Examples of some of the drugs used belonging to different categories are mentioned in Figure 1. They are used as individual or in combination depending upon the severity and type of psoriasis involved. One of the major issues associated with current therapies available is that primarily all of them are associated with side effects when delivered by oral or intravenous route.Methotrexate, a potential antineoplastic agent is prescribed to treat severe and moderate psoriasis. Side effects caused by the systemic use of methotrexate include hepatic toxicity, loss of vision, headache, hair loss, etc. In addition, none of them bring about a permanent remission of psoriasis. Other major shortcomings of conventional dosage forms available are costly treatments which lead to an economic burden, staining of clothes, psychosocial issues, instability and short half-life of drugs leading to shorter duration of action. Another important issue is the lack of targetability resulting in poor bioavailability.8 Moreover, some of these drugs are delivered by topical delivery through skin. Skin itself acts as a barrier hindering the permeation of drugs into its deeper layer. In case of psoriasis conditions, the normal architecture of skin is affected which includes thickened and inflamed skin lesions covered with scales, imbalance of skin lipid, excessive growth and aberrant differentiation of corneocytes, moisture deficit, sensitive, tethered hairy skin. The predisposing factors that limit the bioavailability of conventional formulations through skin are variability in percutaneous absorption due to site, disease, age, etc., irritation potential and other toxicities due to drug, heterogeneity and inducibility of the skin in turnover and metabolism.
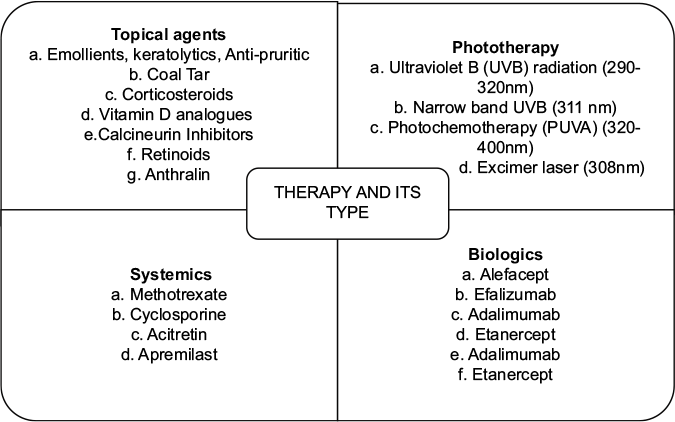
- Classification of a current therapy regimen for psoriasis
Novel Delivery Strategies to Overcome the Shortcomings of Conventional Treatments
Researchers have extensively worked and envisaged various delivery approaches to overcome some of the shortcomings associated with conventional formulations. Nanotechnology has emerged as a promising delivery strategy in recent times to enhance permeation of drug across the skin and improve bioavailability. Some of the advantages offered by nanotechnology are an increase in solubility, dose reduction, improvement of therapeutic effect, prolonged release and improved patient compliance. Various nanocarriers that have been studied are polymeric nanoparticles, solid lipid nanoparticles, nanoliposomes, nanoemulsions and nanohydrogels. The present paper discusses in details formulation aspects of nanocarriers, their design development, herbal drugs, alternate delivery by transungual route and new advances in small interfering RNA and stem cell therapy. Summary of different nanoparticulate drug delivery in psoriasis management is mentioned in Table 1.
| Serial number | Nanoparticulate drug delivery | Active ingredient | Polymer used | Summary | References |
|---|---|---|---|---|---|
| 1 | Nanocapsules | Tretinoin | Polycaprolactone and lipids comprising caprylic/capric triglyceride mixture, sorbitan monostearate as surfactant.Nanocapsules were incorporated in CarbopolUltrez NF 10 based gel | There was increased permeation and photostability of tretinoin. There was enhanced retention of nanocapsulesin human skin | 9 |
| 2 | Liposomes | Calcipotriol | Liposomes were PEGylated | PEGylated liposomes enhanced active targeting through receptor-ligand conjugation to specific tissues | 10 |
| 3 | Deformable liposomes | MTX | Phosphatidylcholine and oleic acid | Oleic acid rendered flexibility and deformability to liposmes. As a result, permeation of MTX was enhanced | 11 |
| 4 | Solid in oil nanocarriers | MTX | Reverse micellar system comprising non-ionic surfactants (ER290, ER190 or L-195) complex. Urea enhanced the solubility of drug. Presence of IPM in formulation controlled particle size to 50–100 nm | Lipophilic nature and nanolevel size of the carrier enhanced the fusion of MTX and stratumcorneumleading to enhanced permeationcapacity. In order to increasethe solubility of drug, it was complexed with amino acids | 12 |
| 5 | Nanogel | MTXbetamethasone dipropionate and salicylic acid | Styrene-divinyl benzene, methyl methacrylate, NIPAM, nonionic monomer butacrylate were used as monomers for formulating nanogel | Change in temperature during application resulted in the structural deformation of nanogel, enhanced penetration of drug. Incorporation of saturatedNa2CO3 enhanced MTX flux from nanogel comprising NIPAM monomers The combination formulation in nanogel form showed changes in epidermal membrane structure. Combination of betamethasone dipropionate along with salicylic acid as microemulsion based hydrogel showed anti-inflammatory activity of about 72% whereas marketed preparation exhibited about 44% activity when tested in carrageenan-induced hind edema model in Wistar rats |
14 |
| 6 | Nanoemulsions | Clobetasol propionate | Eucalyptus oil, tween 20 and ethyl alcohol | Experimental results showed significant increase in NTPDase activity of lymphocytes in clobetasol propionate nanoemulsion treatment group indicating promising activity against allergic contact dermatitis | 15 |
| 7 | Prodispersion liposphere | Cyclosporin | Surfactants, ethyl lactate and lipids | Liposphere formulation showed improved bioavailability of cyclosporin and stability under normal conditions for about 2 years | 16 |
| 8 | SLN | Betamethasone dipropionate/Calcipotriol | Precirol ATO 5 as lipid, PluronicF-68 as surfactant | SLN-gel based formulations showed controlled release for more than 48 h. Dermal bioavailability when tested in human cadaver skin increased 2.8times for calcipotriol and about 5.28times for betamethasone dipropionate when compared with marketed product. When tested inin vitro on human hyperproliferative keratinocyte cell line(HaCaT cells) and in vivo using BALB/c mouse tail model, there was significant decrease in proliferation of cells | 17 |
| 9 | Nail lacquer | Isotretinoin | Eudragit RS. 100, polyethylene glycol, glycerol | Transungual delivery was explored for treatment of nail psoriasis. When tested in bovine hoof as diffusion membrane, the flux increased about 1.72times when compared to marketed product | 18,19 |
SLN: Solid lipid nanoparticles, NIPAM: N-isopropylacrylamide, MTX: Methotrexate
Nanoparticles
Nanoparticles are of two types,nanocapsules and nanospheres. Nanocapsules comprise drug reservoir in core encapsulated in polymeric coat, whereas nanospheres consist of drug dispersed in polymeric matrix. Assessment of therapeutic benefits offered by conventional skincare formulations such as ointments and creams is difficult since drugs delivered through them could be removed easily by contacting, wetting and retention on the skin or in the layers of skin is minimal and for a transient period. Nanocapsule suspensions incorporated in suitable base act as reservoir systems and after application, they can penetrate, accumulate and get retained in the layers of skin for a longer period. Enhanced penetration rate in the skin layers and larger specific surface coverage because of the nanosize are some of the advantages offered by nanoparticles. Nanoparticles provide a homogenous release of encapsulated drugs. Ourique et al. formulated nanocapsules to modify the penetration and ameliorate photostability of tretinoin, a promising antipsoriatic drug.9 The group formulated polymeric nanocapsules comprising lipid and drug core. The polymer used was polycaprolactone and lipids used were caprylic/capric triglyceride mixture along with sorbitan monostearate as surfactant. The nanocapsules were incorporated in Carbopol Ultrez NF 10. The drug entrapment efficiency of nanocapsules was found to be more than 99%, size was about 200–250nm with polydispersity index of less than 0.25. The group performed Franz diffusion studies to confirm the retention of nanocapsules in human skin.
Liposomes
Liposomes are vesicular carriers widely used as topical delivery systems. Liposomes can be varied in their membrane composition, size and cholesterol content. The factors that can enhance penetration of liposomes through stratum corneum are type and concentration of surfactant,size, charge and fluidity. Surfactants present inside the liposome have the ability to break the bilayer and the drug can easily permeate through skin. PEGylated liposomes are commonly used for systemic as well as transdermal delivery of drugs. Calcipotriol is a potent drug used to treat psoriasis by inhibiting the proliferation of keratinocytes. The drug being an analogue of vitamin D3 undergoes conjugation with the Vitamin D receptor present in the epidermis of skin. This specification of calcipotriol can be achieved by efficient drug delivery using PEGylated liposomes that can target the drug to the lower layer of epidermis.
Stability of PEGylated liposomes was achieved with hydrophilic polymers, i.e., polyethylene glycol (PEG). PEGylated liposomes could increase the in vivo stability by restricting interaction with plasma proteins. Some alterations in the drug release profile could be obtained due to the presence of polymer. One of the major applications of PEGylated liposomes was its active targeting through receptor-ligand conjugation to specific tissues.10
Deformable liposome
Deformable liposomes of methotrexate were prepared by entrapping oleic acid in liposomes.11 Improved skin delivery of drugs by this novel drug delivery system was the major advantage which was influenced by enhanced penetration and permeation of vesicles into stratum corneum under nonocclusive conditions. Deformable liposomes have been highly useful for the formulation of lipophilic cosmetic products. The physicochemical properties, e.g., in vitro release and permeability characteristics of deformable liposomes, have been investigated. Phosphatidylcholine and oleic acid were the two main substances used for the preparation of deformable liposomes. Oleic acid conferred elasticity and therefore enhanced permeability of liposomes. Deformable liposomes exhibited enhanced loading and entrapment efficiency and high penetration capability and diffusivity through porcine skin as compared to conventional liposomes.
Solid in oil nanocarriers
Solid in oil nanocarriers were used as the vehicles for the transdermal delivery of methotrexate. Yang et al. have conducted studies on solid in oil nanosuspensions which were a kind of oil-based nanocarriers.12 Here, suspension type nanocarrier was introduced as a reverse micellar system into which methotrexate coated nonionic surfactants (ER290, ER190 or L-195) complex was incorporated. The oil-based nanocarriers helped in the penetration of methotrexate through stratum corneum. Compared to other conventional drug delivery systems such as liposomes, niosomes,etc.,the solid in oil nanocarriers possess higher penetration capacity. Due to the lipophilic nature and nano level size of the carrier, it could enhance the fusion of methotrexate and stratum corneum which enhanced the permeation capacity.
In that study, to increase the solubility of the drug, a complex of amino acids and methotrexate was prepared and enhanced stability was achieved by introducing the surfactant coating technique. Solid in oil suspension nanocarriers could accommodate multidrugs such as methotrexate, amino acids and urea. Urea had an important role in the release mechanism of methotrexate from the nanocarriers. Uniform size distribution was achieved by the addition of isopropyl myristate (50–100nm). The advantages offered by solid in oil suspension nanocarriers were multidrug accumulating capacity, enhanced permeation capacity, dispersibility, stability and uniformity of size.
Nanogel
Nanogel comprises nanoparticles incorporated in hydrogel. Hydrogels are composed of crosslinked hydrophilic polymer network made from synthetic polymers or biopolymers which are chemically or physically crosslinked.
Singka et al. developed nanogels for the topical delivery of methotrexate. The size of nanogels particles varied from 100 nm to 1 μm in size.13 The swelling nature of the colloidal particles in many solvents was one of the reasons for the wide application of this delivery. The monomers used in nanogels were having vital importance in volume phase transition depending on various stimuli such as temperature, solvent type and ionic strength. Styrenedivinyl benzene, methyl methacrylate, etc. could be used for the production of nanogels. The most widely used monomer is N-isopropylacrylamide since it could collapse at low temperature (32°C–34°C). In the study, the nanogel was prepared with copolymer of N-isopropylacrylamide and nonionic monomer butacrylate.
On application, the nanogel underwent deswelling and the expulsion of methotrexate was influenced by a change in temperature during penetration through skin. The incorporation of saturated Na2CO3-enhanced methotrexate flux from nanogel and by the use of N-isopropylacrylamide monomers, the biosynthesis of polyethylene glycol-2, an inflammation mediator, was reduced.
Hydrogels produced by the crosslinking of polymer chains led to the formation of polymer gel with high molecular weight. Hydrogen bonding is the main mechanism by which a large amount of water is entrapped by hydrogel.
Hydrogels were used to increase the effectiveness of topical delivery of methotrexate by iontophoretic delivery. The effectiveness of delivery of methotrexate by iontophoretic mechanism depended on the type of hydrogel used and the amount of drug loaded into it.
Baboota et al. explored the application of nanocarrier hydrogels for the topical delivery of betamethasone dipropionate for therapy of psoriasis.14 Incorporation of salicylic acid into this system enhanced the descaling of skin and stability of formulation. Researchers performed histopathological studies on skin used for permeation studies. Preliminary examinations showed considerable changes in epidermal membrane structure. In addition, antiinflammatory studies were performed on Wistar rats using carrageenan-induced hind edema method. Combination of betamethasone dipropionate along with salicylic acid as microemulsion based hydrogel showed antiinflammatory activity of about 72%, whereas marketed preparation exhibited about 44% activity.
Nanoemulsion
Nanoemulsions are novel formulations forthe topical delivery of drugs. The main advantage of nanoemulsion is that it can deliver poorly water-soluble drugs into the bottom of skin layer. The absorption rate of drug can be increased since the drug particles are solubilized in internal dispersed phase generally oil and the globules of dispersed phase are reduced to submicron size (up to 10–200 nm). Nanoemulsions are feasible with respect to industrial scalability and controlled release of the drug at the target site. These are some of the peculiar advantages rendered by the system.
Alam et al. formulated oil in water nanoemulsions of clobetasol propionate for prolonged delivery for treatment of psoriasis.15 The researchers evaluated the potential of developed formulation for the treatment of atopic dermatitis and psoriasis. The nanoemulsion also contained eucalyptus oil along with tween 20 and ethyl alcohol. In vivo studies conducted involved skin irritation, inflammatory and nickel-induced contact dermatitis. It was observed that there was a significant increase in nucleoside triphosphate diphosphohydrolase (NTPDase) activity of lymphocytes in the clobetasol propionate nanoemulsion treatment group, indicating promising activity against allergic contact dermatitis. The formulation also showed antiinflammatory activity without any skin irritation. Studies have shown that nanoemulsion can be a viable option to deliver antipsoriatic drug topically.
Prodispersion liposphere
Prodispersion formulations are novel drug delivery systems composed of solid fat, amphiphilic solvents and dispersing agents. Cyclosporin is an important drug widely used to treat psoriasis and has the ability to suppress immune reactions by interfering in the growth of T cells.
Avramoff et al. prepared prodispersionliposphere for the delivery of lipophilic drug cyclosporine.16 In this formulation, a homogeneous solution was prepared containing cyclosporine, surfactants, ethyl lactate and lipids. The lipidic solution was added to aqueous medium to form dispersion. The lipospheres were characterized for particle size and in vitro drug release using cryotransmission electron microscopy, differential scanning calorimetry and ultracentrifugation to ascertain the formation and stability. Liposphere formulation showed improved bioavailability of cyclosporin and stability under normal conditions for about two years.
Solid lipid nanoparticles
Solid lipid nanoparticles are classified as solid lipid core matrix spherical particles with an average diameter between 10 and 1000 nm. Solid lipid nanoparticles incorporate lipophilic molecules.17 Lipid core materials comprise triglycerides (e.g., tristearin), diglycerides (e.g., glycerol bahenate), monoglycerides (e.g., glycerol monostearate), fatty acids (e.g., stearic acid), steroids (e.g., cholesterol) and waxes (e.g.,cetylpalmitate)stabilized by surfactants (emulsifiers). Sonawane et al. prepared solid lipid nanoparticles comprising betamethasone dipropionate and calcipotriol by hot melt high shear homogenization technique for combination therapy of psoriasis. The formed solid lipid nanoparticles were then added to Carbopol 980 based gel. Precirol ATO 5 was used as lipid and Pluronic F-68 as surfactant. Solid lipid nanoparticles were evaluated for entrapment efficiency, drug release and in vitro skin permeation using Franz cell and dermatopharmacokinetic study. In vitro drug release study yielded prolonged release of about less than 50% at the end of 48 h. Nondetectable quantity of drugs obtained in receptor medium confirmed maximum retention of solid lipid nanoparticles in the skin. Dermal bioavailability increased about 4.12 times for calcipotriol and about 10.47 times for betamethasone dipropionate in case of solid lipid nanoparticles-based gel in comparison with marketed formulation in rat skin. For human cadaver skin, dermal bioavailability increased about 2.8 times for calcipotriol and about 5.28 times for betamethasone dipropionate in case of solid lipid nanoparticles-based gel in comparison with marketed formulation in rat skin. Distribution of solid lipid nanoparticles in different layers of skin was evaluated using confocal laser scanning microscopy.Microscopy studies indicated transfollicular pathway for penetration ofdrugs into the skin. The antipsoriatic potential of solid lipid nanoparticles was tested in vitro by checking the effect on proliferation of human hyperproliferative keratinocyte cell line (HaCaT cells) and in vivo using BALB/c mouse tail model. The treatment tail portions were subjected to histopathological studies and were evaluated for epidermal thickness and melanocyte count. Solid lipid nanoparticle-based gel showed significant inhibition of hyperproliferative epidermal cells in comparison to conventional formulations and in case of tail model studies, a decrease in epidermal thickness and an increase in melanocyte count indicate its promising antipsoriatic activity. The group concluded that solid lipid nanoparticle-based gel containing combination therapy can be further translated into commercial product after clinical trials.
Nail lacquer
Treating nail psoriasis has been extremely difficult and only a few therapy regimens are available in clinical practice. Research shows that up to 55% of people with psoriasis may have nail involvement. In many cases, symptoms of nail psoriasis, like pitting and crumbling, can also be a warning sign and beginning of psoriatic arthritis. Topical treatments with 0.1% cream and gel of tazarotene and 0.1% tacrolimus ointment are employed however have limited access across nail plate therefore show low efficacy. Researchers have explored the potential of transungual delivery for improving treatment of nail psoriasis. Joshi et al. developed isotretinoin-based nail lacquer.18 In the experimental trials, thioglycolic acid and eugenol showed maximum hydration enhancement factor and permeation enhancement. Other formulation excipients included were Eudragit RS 100 as film former and release retardant, polyethylene glycol and glycerol. Ex vivo permeation studies were performed using bovine hoof as diffusion membrane. Target flux of the developed nail lacquer was found to be 176 μg/cm2/h in comparison to marketed product which showed 95.5 μg/cm2/h which means 1.72 times higher indicating enhanced drug permeation. Other drugs that are being envisaged in various clinical trials are cyclosporine 5% and vitamin D-based treatment calcipotriene 0.005%.19
Herbs/herbal phytoconstituent for psoriasis
Herbal drugs have been used as traditional medicines for thousands of years for treatment as well as for alleviation of various diseases. They have been used traditionally in various systems like Ayurveda, Unani and Traditional Chinese Medicine. Herbal drugs or phytoconstituents are now being considered as promising alternatives to drugs of synthetic origin. Herbal phytoconstituents derived from traditional plants have been transformed into novel delivery carriers [Table 2].20-24
| Bioactives | Novel carrier | Potential effects | Reference |
|---|---|---|---|
| Psoraleacorylifolia | Microemulsion gel | Improved stability | 20 |
| Curcuminoids | Nanoparticles | Enhanced skin uptake, better retention | 21 |
| Glycyrrhetic acid | Nanoemulsion | Increased stability of formulation, better transdermal effect | 22 |
| Tea tree oil | Microemulsion | Potential in enhanced drug uptake | 23 |
| Colchicine | Liposome | Sustained delivery | 24 |
Recent Novel Advancements and Drug Delivery Strategies
Gene therapy approach involves identifying a specific functional gene or specific fragment and replacing it in place of a defective gene for the treatment of diseases originating due to genetic disorder. Mutation of the gene that codes enzyme adenosine deaminase results in disorder of normal gene sequence.25
Once the replacement gene is identified, the next step involved is inserting it into an appropriate site within the genome. The crucial step involved in this exercise is to ascertain the activity.
Al-Raawi et al. in their study identified the role of N-JARID2, a fragment of JARID2, in maintaining the normal architecture of skin. The group found that the protein N-JARID2 is responsible to maintain skin cells in their normal state of differentiation and this can have potential application in treatment of psoriasis.26
Posttranscriptional gene silencing process by RNA interference involves degradation of specific messenger RNA in the cell cytoplasm by small interfering RNA molecules. This strategy has attracted significant attention since the resultant reduction in protein expression is applicable to several classes of molecular targets, representing a great promise for silencing disease-promoting genes. Skin disorders related to inflammation (such as psoriasis,vitiligo and atopic dermatitis), abnormal cell behavior(squamous cell carcinoma and melanoma) and damage (burn and wound) as well as monogenetic skin disorders (pachyonychiacongenita) have been considered suitable for small interfering RNA therapy due to the existence of well-defined molecular targets that can be silenced, resulting in therapeutic benefits. Topical administration of small interfering RNA to the skin would be feasible due to the accessibility of the site, ease of administration and possibility to overcome the first-pass metabolism. However, the skin outermost layer, the stratum corneum, represents an efficient barrier against the entry of substances into the skin.
To overcome the limitations related to size, rapid enzymatic degradation and strong anionic charge of the phosphate backbone of small interfering RNA molecules (with consequent electrostatic repulsion from the anionic cell membrane surface), novel delivery strategies are employed. Nonviral vectors, nanocarriers, small interfering RNA complexes with positively charged compounds (e.g., cationic polymers, lipids,dendrimers and peptides or proteins), conjugates with small molecules (e.g., cholesterol), antibodies, polymers and lipids have been shown promising results in small interfering RNA delivery to knock down specific targets in different cell lines and tissues.27-41
Stem cell therapy
Mesenchymal stem cells are multipotent adult stem cells which have the unique capability of proliferating for a prolonged period of time and remain in undifferentiated form. The daughter cells generated from them can further differentiate into cells of host tissues, thus helping in the repair process. Mesenchymal stem cells have a promising role in cell-based therapy due to their tissue regenerative and host immune-modulatory capabilities.42 Mesenchymal stem cells possess unique immunomodulatory properties which make them a promising tool for the treatment of various inflammatory diseases. Based on accumulated results, mesenchymal stem cells obtained from patients with psoriasis have been shown to have impaired antiinflammatory function against the cell subsets.Previous preclinical study demonstrated that subcutaneous infusion of human umbilical cord blood-mesenchymal stem cells efficiently attenuates imiquimod-induced psoriasis-like skin inflammation in mice by suppressing Th1, Th2 and Th17 differentiation and up-regulating Treg population. Psoriatic mice administered with mesenchymal stem cells exhibited the decreased skin reactive oxygen species level and immune cell infiltration into the skin lesions. Moreover, the therapeutic effect of mesenchymal stem cells can be remarkably enhanced by the overexpression of superoxide dismutase 3, a strong antioxidant enzyme. According to the NIH ClinicalTrials.gov database, the only clinical trial (NCT02491658) is currently underway using umbilical cord mesenchymal stem cells for patients with moderate-to-severe psoriasis vulgaris. Based on early results of this clinical study, two patients infused with umbilical cord mesenchymal stem cells remained relapse-free of psoriasis for 4 to 5 years.43-45
Conclusion
Psoriasis is an atypical systemic autoimmune disease with unknown etiology primarily affecting skin further propagating to nails and joints leading to arthritis. Despite advancement in medical science, complete cure of this disease is not yet established. Thus, the quality of life after disease identification is definitely compromised in a big way and requires a conscious efforts and protocols as disease management is an important tool to keep this disease under control. Topical therapy whether it is conventional or novel is always a choice of delivery system for pharmaceutical technocrats. The large range of symptoms and comorbidities associated with psoriasis makes it imperative to treat this disease, along with other underlying diseases. A proper evaluation of psoriasis types should be done at initiation in order to form a tailored medication regimen. The treatment options chosen for patients should always be individualized due to the potential consequences of these medications. With the emergence of biologics and the development of new drugs, psoriasis treatment is rapidly changing. Increasing awareness of the public and health care professional will allow for more informed decisions about treatment options.
Further research in psoriasis is needed to define exact causes of the disease in order to manufacture drugs better tailored for medication regimens. Research on newer methods to develop optimized drug delivery through novel carriers are in progress to minimize the cost and increase efficacy as well as minimize side effects. The challenge that is offered by topical treatment is the presence of stratum corneum as a barrier. Conventional forms of drug delivery through skin encounter many side effects and other application difficulties. Disruption of stratum corneum and targeting to the deeper layers of skin are not possible with conventional ointments, creams, etc. Hence, novel dermal delivery systems help to overcome these limitations, thereby enhance the bioavailability and potential of drug and are widely being studied; recent innovations and development in the field of biotechnology along with pharmaceutical carrier and delivery system have a promisingly potential role in the diagnosis, mitigation, management and treatment of psoriasis and psoriasis-like diseases.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Psoriatic and Reactive Arthritis : A Companion to Rheumatology Amsterdam: Mosby, Elsevier; 2007. p. :4.
- [Google Scholar]
- Psoriasis epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26:314-20.
- [CrossRef] [PubMed] [Google Scholar]
- Cause 1990-2010 R by. Global Burden of Disease Study. Institute for Health Metrics and Evaluation.
- [Google Scholar]
- Cost-of-illness in patients with moderate and severe chronic psoriasis vulgaris in German. J Dtsch Dermatol Ges. 2005;2005:511-8.
- [CrossRef] [PubMed] [Google Scholar]
- Why statistics matter: Limited inter-rater agreement prevents using the psoriasis area and severity index as a unique determinant of therapeutic decision in psoriasis. J Invest Dermatol. 2012;132:2171-5.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology of psoriasis. Indian J Dermatol Venereol Leprol. 2013;79(Supp l7):S1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Novel drug delivery systems in topical treatment of psoriasis: Rigors and vigors. Indian J Dermatology Venereol Leprol. 2010;76:612.
- [CrossRef] [PubMed] [Google Scholar]
- Improved photostability and reduced skin permeation of tretinoin: development of a semisolid nanomedicine. Eur J Pharm Biopharm. 2011;79:95-101.
- [CrossRef] [PubMed] [Google Scholar]
- Calcipotriol delivery into the skin with PEGylated liposomes. Eur J Pharm Biopharm. 2012;81:532-9.
- [CrossRef] [PubMed] [Google Scholar]
- Physico-chemical characteristics of methotrexate-entrapped oleic acid-containing deformable liposomes for in vitro transepidermal delivery targeting psoriasis treatment. Int J Pharm. 2012;427:426-34.
- [CrossRef] [PubMed] [Google Scholar]
- Transdermal delivery of the anti-rheumatic agent methotrexate using a solid-in-oil nanocarrier. Eur J Pharm Biopharm. 2012;82:158-63.
- [CrossRef] [PubMed] [Google Scholar]
- Enhanced topical delivery and anti-inflammatory activity of methotrexate from an activated nanogel. Eur J Pharm Biopharm. 2010;76:275-81.
- [CrossRef] [PubMed] [Google Scholar]
- Nanocarrier-based hydrogel of betamethasone dipropionate and salicylic acid for treatment of psoriasis. Int J Pharm Investig. 2011;1:139-47.
- [CrossRef] [PubMed] [Google Scholar]
- In vivo study of clobetasol propionate loaded nanoemulsion for topical application in psoriasis and atopic dermatitis. Drug Invent Today. 2013;5:8-12.
- [CrossRef] [Google Scholar]
- Cyclosporin pro-dispersion liposphere formulation. J Control Release. 2012;160:401-6.
- [CrossRef] [PubMed] [Google Scholar]
- Solid lipid nanoparticles-loaded topical gel containing combination drugs: An approach to offset psoriasis. Expert Opin Drug Deliv. 2014;11:1833-47.
- [CrossRef] [PubMed] [Google Scholar]
- Matrix based system of isotretinoin as nail lacquer to enhance transungal delivery across human nail plate. Int J Pharm. 2015;478:268-77.
- [CrossRef] [PubMed] [Google Scholar]
- Nail Psoriasis: A Review of Treatment Options. Drugs. 2016;76:675-705.
- [CrossRef] [PubMed] [Google Scholar]
- Herbs: A potential source for the development of new pytomedicinals. Pharma Rev. 2003;14:59-63.
- [Google Scholar]
- Development of curcuminoids loaded poly(butyl) cyanoacrylate nanoparticles: Physicochemical characterization and stability study. Eur J Pharm Sci. 2009;37:395-404.
- [CrossRef] [PubMed] [Google Scholar]
- Nanoemulsions as vehicles for topical administration of glycyrrhetic acid: Characterization and in vitro and in vivo evaluation. Drug Deliv. 2010;17:123-9.
- [CrossRef] [PubMed] [Google Scholar]
- Microemulsion based transdermal drug delivery of tea tree oil. Int J Drug Dev Res. 2011;3:191-8.
- [Google Scholar]
- Elastic liposomal formulation for sustained delivery of colchicine: In vitro characterization and in vivo evaluation of anti-gout activity. AAPS J. 2009;11:54-64.
- [CrossRef] [PubMed] [Google Scholar]
- What is Gene Therapy? Genetics Home Reference NIH. Available from: https://ghr.nlm.nih.gov/primer/therapy/genetherapy [Last accessed on 2019 Dec 23]
- [Google Scholar]
- A novel form of JARID2 is required for differentiation in lineage-committed cells. EMBO J. 2019;38:e98449.
- [CrossRef] [PubMed] [Google Scholar]
- Hydrophobization and bioconjugation for enhanced siRNA delivery and targeting. RNA. 2007;13:431-56.
- [CrossRef] [PubMed] [Google Scholar]
- Knocking down barriers: Advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129-38.
- [CrossRef] [PubMed] [Google Scholar]
- Drug delivery of siRNA therapeutics: Potentials and limits of nanosystems. Nanomedicine. 2009;5:8-20.
- [CrossRef] [PubMed] [Google Scholar]
- Delivery of siRNA therapeutics: Barriers and carriers. AAPS J. 2010;12:492-503.
- [CrossRef] [PubMed] [Google Scholar]
- siRNA delivery: From lipids to cell-penetrating peptides and their mimics. Chem Biol Drug Des. 2012;80:787-809.
- [CrossRef] [PubMed] [Google Scholar]
- Development of RNAi technology for targeted therapy-A track of siRNA based agents to RNAi therapeutics. J Control Release. 2014;193:270-81.
- [CrossRef] [PubMed] [Google Scholar]
- Antisense oligonucleotide treatments for psoriasis. Expert Opin Biol Ther. 2004;4:75-81.
- [CrossRef] [PubMed] [Google Scholar]
- Amelioration of psoriasis by anti-TNF-alpha RNAi in the xenograft transplantation model. Mol Ther. 2009;17:1743-53.
- [CrossRef] [PubMed] [Google Scholar]
- Noninvasive delivery of siRNA into the epidermis by iontophoresis using an atopic dermatitis-like model rat. Int J Pharm. 2010;383:157-60.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting (V600E) B-Raf and Akt3 using nanoliposomal-siRNA inhibits cutaneous melanocytic lesion development. Cancer Res. 2008;68:7638-49.
- [CrossRef] [PubMed] [Google Scholar]
- Topical matrix-based siRNA silences local gene expression in a murine wound model. Gene Ther. 2007;14:1305-8.
- [CrossRef] [PubMed] [Google Scholar]
- Single-nucleotide-specific siRNA targeting in a dominant-negative skin model. J Invest Dermatol. 2008;128:594-605.
- [CrossRef] [PubMed] [Google Scholar]
- Lipid-mediated gene delivery to the skin. Eur J Pharm Sci. 2011;43:199-211.
- [CrossRef] [PubMed] [Google Scholar]
- Delivery systems and local administration routes for therapeutic siRNA. Pharm Res. 2013;30:915-31.
- [CrossRef] [PubMed] [Google Scholar]
- In vivo gene silencing following non-invasive siRNA delivery into the skin using a novel topical formulation. J Control Release. 2014;196:355-62.
- [CrossRef] [PubMed] [Google Scholar]
- Mesenchymal stem cell conditioned media ameliorate psoriasis vulgaris: A case study. Case Rep Dermatol Med. 2019;2019:8309103.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphocyte inhibition is compromised in mesenchymal stem cells from psoriatic skin. Eur J Dermatol. 2014;24:560-7.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization and profiling of immunomodulatory genes in resident mesenchymal stem cells reflect the Th1-Th17/Th2 imbalance of psoriasis. Arch Dermatol Res. 2014;306:915-20.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of Psoriasis with Mesenchymal Stem Cells. Am J Med. 2016;129:e13-4.
- [CrossRef] [PubMed] [Google Scholar]






