Translate this page into:
Onychomycosis: Newer insights in pathogenesis and diagnosis
Correspondence Address:
Chander Grover
House No.420 B, Pocket II, MayurVihar, Phase I, Delhi - 110 091
India
| How to cite this article: Grover C, Khurana A. Onychomycosis: Newer insights in pathogenesis and diagnosis. Indian J Dermatol Venereol Leprol 2012;78:263-270 |
Abstract
Onychomycosis (OM) is the commonest disorder affecting the nail unit. The fact that it affects 3-26% people worldwide goes to show that it is a significant health problem. The prevalence of OM has been reported to be increasing over the years. Although, we know much about various predisposing factors, we are yet unclear about its exact pathogenesis. The peculiarities of the nail unit with respect to its structure and its immune mechanisms make OM an adversary, which once established is difficult to eradicate. There have been many recent advances in our understanding of the pathogenesis of OM and our methods of diagnosing it. The increasingly valuable role of histopathology; refinements in its technique; PCR techniques; Optical coherence tomography and advances in spectrometric techniques have been reported. The present review is aimed at discussing the newer advances in our understanding of the pathogenesis of various clinical types of OM apart from the newer and exciting techniques of diagnosing it.Introduction
Onychomycosis (OM) is one of the commonest nail conditions encountered in dermatological practice. Prevalence rates for OM varying from 3 to 5% have been found in most studies; however, a few reports suggest a higher prevalence of even up to 26% in the general population. [1] The prevalence of onychomycosis seems to vary across the world because of various socioeconomic and cultural factors. [2] Various Indian studies have also reported an incidence in the range of 0.5-5% in the general population. [3],[4]
A variety of fungi have been implicated in the causation of OM. Most commonly, the source of infection is the infected surrounding skin and the same organism is detectable from both sites. The Achilles foot project (covering 80,396 patients from East Asia and Europe), found dermatophytes to be the most common causative organisms for OM, accounting for about 68% of all cases. This was followed by yeasts (11%) and non-dermatophyte molds (NDM) (11% cases). [1] In this series, 0.1% cases had mixed infections and no mycological growth was recordable from the rest 10.9% cases. [1] Among the dermatophytes, the most common organism reported is Trichophyton rubrum (53% cases) followed by T. mentagrophyte var interdigitale (13%), Epidermophyton floccossum (1.2%) and Microsporum species. [1] Yeasts generally invade already damaged nails (like in chronic paronychia) or nails in immunosuppressed (as in chronic mucocutaneous candidiasis). Candida albicans is the most common yeast responsible (8%) followed by C. parapsilosis (1.2%). [1] Among NDM, the commonly isolated species are Aspergillus (4%) and Scopulariopsis brevicaulis (3%). [1] Although, there is still much debate as to whether most NDMs actually cause nail infection or they are mere laboratory contaminants or secondary invaders of already damaged nails, the accumulating evidence highly suggests that some NDMs can be true primary invaders. [5]
The present article focuses on the newer and exciting advances in our understanding of the pathogenesis of OM. Newer techniques enabling accurate and sensitive diagnosis of the condition are also discussed.
Pathogenesis
Invasion of nail apparatus by fungi is a less studied area. However, factors involved in the fungal invasion of skin has been relatively well studied and occurs in several stages. [6],[7] Both mechanical and chemical factors have a role to play in the entire process. The essential steps are surface adhesion followed by invasion into the sublayers. The site and pattern of invasion which leads to production of different clinical types of OM can be seen from [Figure - 1]. Nail involvement occurs by penetration of fungal elements and secretion of enzymes that degrade the skin components. Dermatophytic fungi have been shown to have keratinolytic, proteolytic and lipolytic activities. [6],[7] The hydrolysis of keratin by proteinases not only facilitates invasion into tissues, but also provides nutrition to the fungi. Nails being skin appendages undergo essentially the same pattern of invasion. However, the nail apparatus has some unique features to offer.
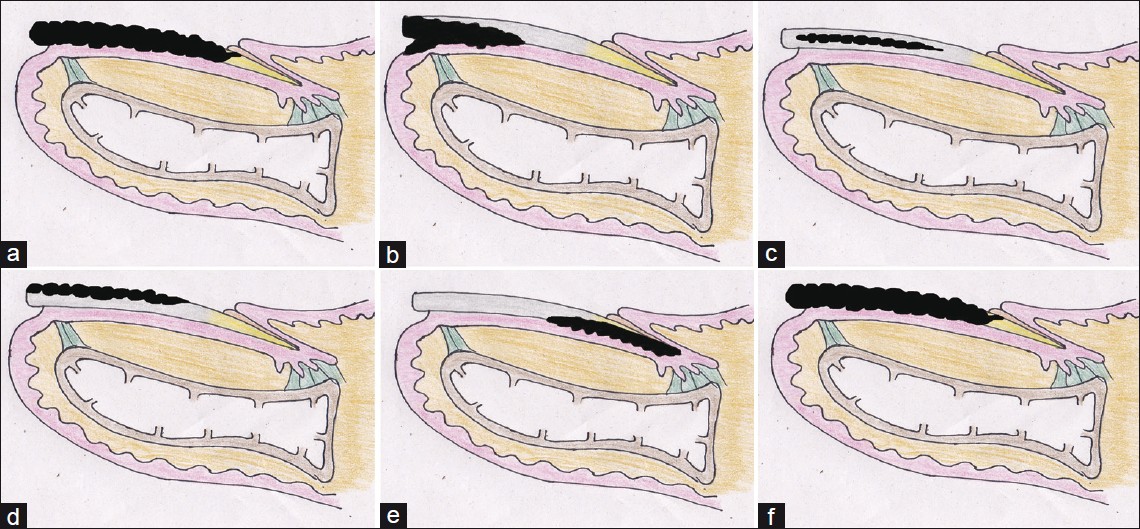 |
| Figure 1: Pathogenesis of Onychomycosis (a) Anatomical structure of the normal nail unit. (b) Pattern of fungal invasion in distal lateral subungual onychomycosis. (c) Pattern of fungal invasion in endonyx onychomycosis. (d) Pattern of invasion in superficial white onychomycosis. (e) Pattern of invasion in PSOM. (f) Fungal involvement in a case of TDOM |
Our knowledge of the immune mechanisms operative in the nail apparatus is still preliminary. Structurally, the nail apparatus is exposed to a harsh environment and is prone to damage and invasion by various organisms. The unique anatomy of the nail is prone to provide easy portals of entry to pathogens, through the proximal nail fold and the distal nail fold. However, these are physically protected by the cuticle and the distal solehorn, respectively [Figure - 1]a. The nail apparatus has certain essential immunological differences as compared to that of skin. The nail unit is isolated from the body′s cell-mediated immunity (CMI). [8] It is a site of relative immune privilege due to a very low level of expression of MHC (Major histocompatibility) Class 1a antigens, local production of potent immunosuppressive agents, dysfunction of antigen presenting cells (APC) and inhibition of Natural Killer (NK) cell activity. [8] Also, dermatophytes are strongly keratinophilic organisms. This is because of their ability to form perforating organs due to which they quickly digest keratin. [9] On the other hand, there are various protective mechanisms at play as well. Nail unit has been shown to possess a strong innate immunity. [10] An increased local expression of antimicrobial peptide (human cathelicidin LL-37) has been shown in the study by Dorschner et al. [10] Cathelicidin LL-37 is not expressed in human skin under normal circumstances, but it gets induced upon exposure to infection or inflammation. However, it is strongly expressed in the nail unit. Being a soluble antimicrobial, it has been shown to have potent activity against Pseudomonas aeruginosa[11] and Candida albicans. [10] In addition, a differential distribution of immune cells has been observed in different parts of the nail apparatus. There is a high density of CD4+ cells in the proximal nail fold (PNF) and very low density in the proximal nail matrix (PNM). [12] CD8+ T cells are rarely seen in and around PNF, nail bed, and PNM. The density of Langerhans cells is higher in epithelium of the PNF and nail bed as compared to that of the nail matrix. [12] The Langerhans cells and macrophages in the nail matrix are functionally impaired with respect to their capability of antigen presentation. [1]
In short, due to a lack of effective cell-mediated immunity, the nail apparatus is susceptible to invasion by fungal organisms, if it gets exposed due to various predisposing factors. Hence, OM is usually a chronic infection not associated with inflammation. The nail plate offers an ideal ecological niche for fungal organisms where they can persist for long durations. The presence or role of other mechanisms contributing to elimination of dermatophytes from nail plates is not well known. Any physical compromise of the protective and self-containing structure of the nail apparatus exposes it to an early invasion by fungi which are then very difficult to eradicate.
Physical restrictions to the microbial invasion of nail also get compromised under certain circumstances. Various predisposing factors include vascular diseases, atopy, obesity, diabetes and sports. [1] In the series by Kaur et al., most patients with Candidal OM were involved in occupations that predispose to repeated minor trauma or were engaged in domestic activities which involve wet work. [13] It is known that most dermatophyte species affect the ventral and middle layers of the nail plate, where the keratin is comparatively soft, and in close proximity to the underlying living cells. On the ventral surface, the junctions between cells are more flexible than the tight junctions in the dorsal part. The ventral surface has a highly irregular topography with parallel grooves and ridges providing excellent channels for hyphae to penetrate the nail plate. [14] Also, the intercellular junctions in the ventral plate are more flexible than the tight junctions in the dorsal nail plate. The intermediate layer is involved less commonly, while the dorsal nail plate is rarely involved except in case of white superficial onychomycosis. [15] The dorsal nail plate is the hardest part and has increased calcium content.
Species differences in fungal pathogenecity have also been reported with Trichophyton mentagrophytes being a more active destroyer than Trichophyton rubrum. [15] This active pathogenecity for the nail could be a result of mechanical [16] or enzymatic processes. [17]
In vitro models have been used to illustrate the nail invasion by dermatophytic species. Rashid et al. incubated nail plate fragments with T. mentagrophytes, without any growth nutrients, and conducted serial observations using scanning electron microscopy. [14] The arthroconidia for inoculation were prepared from 10 day old cultures of strain 126 of T. mentagrophytes. The separated and viable arthrospores were capable of showing germ tube formation within 16 hours at 37°C. [14] It was observed that after 6 hours of incubation, arthroconidia start adhering to corneocytes on the ventral nail surface, singly, in pairs, or in clusters. [14] The first evidence of arthroconidial germination is also evident at this time. By 16 hrs, small germ tubes form on the ventral surface, with side branches. By 24 hrs, small microcolonies start forming on the ventral surface, with the tips of the germ tubes penetrating the crevices present on the ventral aspect of nail plate. By 48 hours, a mature mycelium is usually appreciated. [14] Hyphal branches can be seen arising from and penetrating the corneocytes. Penetration of the dorsal nail surface occurs between and through the corneocytes with hyphae insinuating themselves between the corneocytes. [14] On gross examination of the nail fragments, first visible fungal growth was noticed on the 4 th -6 th day. The growth was initially slow and gradually increased thereafter. There was softening of nail fragments by the 14 th day, degeneration by 21 days and almost complete disappearance of the nail plate by 4 weeks. [14]
This pattern of invasion, if correlated clinically, can be seen to give rise to different clinical types of OM [Figure - 1]b-f.
Distal lateral subungual onychomycosis (DLSOM): In this commonest clinical type of OM, the keratin of the hyponychium becomes infected first. The infection then progresses to involve the nail bed and subsequently the nail plate [Figure - 1]b. [18] The infecting organism migrates proximally through the nail plate (against the tide of growth of the nail plate). Mild inflammation with focal parakeratosis, subungual hyperkeratosis and onycholysis, thickening or distortion of nail plate is seen. This type is usually caused by dermatophytes, especially T. rubrum[19] and less commonly T. mentagrophytes, T. tonsurans, and E. floccosum. Toenails are affected more commonly than fingernails. Frequent accompaniments of tinea pedis with toenail involvement and tinea mannum are almost always with fingernail involvement.
Endonyx onychomycosis (EOM): In this variant, there is a primary and exclusive attack on the nail plate with the fungus growing between the nail plate lamellae [Figure - 1]c. This pattern of invasion is specific for T. soudanense (and possibly T. violaceum) and may reflect its high affinity for hard keratins. [20] Clinically, EOM is seen as a diffuse milky-white discoloration of the affected nail, forming irregular wide waves with pits and lamellar splits, with an absence of nail bed hyperkeratosis or onycholysis. [20],[21] Nail plate surface and nail thickness are normal. A large number of fungal hyphae are visible within the nail plate with absence of fungal elements in the nail bed and hyponychium. [20]
Superficial white onychomycosis (SWOM): This is a rare variant where the dorsal part of the nail plate is the initial site of invasion where the fungus causes small superficial white patches, which may coalesce and cover the entire nail plate [Figure - 1]d. It is fairly rare and mostly limited to toenails. In the majority of the cases, the isolated organism is T. mentagrophytes. [22] Occasionally, non-dermatophyte moulds such as Aspergillus terreus, Fusarium oxysporum, or Acremonium spp. has been reported. In HIV patients, SWO has been documented in fingernails as well, and is generally caused by T. rubrum. [23]
Proximal white subungal onychomycosis (PWSOM): T. rubrum is the commonest causative agent. [24] Clinically, PWSOM presents as leukonychia, proximal onycholysis, subungual hyperkeratosis and destruction of the proximal nail plate. It may also present as a pattern of proximal to distal longitudinal leukonychia affecting a single digit, an isolated transverse leukonychial band, or multiple transverse bands separated by areas of normal nail. [25] This pattern has been described in both finger and toenails. The organism first invades stratum corneum of the proximal nail fold and then penetrates to the matrix and the undersurface of the nail plate [Figure - 1]e. [25] However, recent reports have revealed that PWSOM can also present a rapidly developing disease, in which several nails are affected in the course of a few days, especially in the setting of HIV-associated immunosuppression. [26] A probable role of lymphatic spread, endogenous reactivation or auto-reinfection from a deeper site seems more plausible than any new external infection being acquired through the proximal nail fold. [26]
Total dystrophic onychomycosis (TDOM): This type presents as total destruction of the entire nail apparatus including whole thickness of the plate, the nail bed and matrix. The involved nail becomes thick, dystrophic and crumbles down [Figure - 1]f. [18] It may be primary e.g. in cases with chronic mucocutaneous candidiasis or secondary to any of the four previous forms.
Diagnosis
OM has distinct clinical presentation, however, the key features are shared with other onychopathies like psoriasis and lichen planus. Hence, clinical examination in isolation is seldom sufficient to make a diagnosis of OM. It has been shown that of all the onychopathies suspected of being OM, only about half are mycologically positive. [27] In the past, researchers have tried to assess the diagnostic accuracy of individual clinical findings. Fletcher et al., found that four parameters significantly related to positive mycology results were: a history of tinea pedis in the last year; scaling on one or both soles, white crumbly patches on the nail surface, and an abnormal color of the nail plate. [28] Doval et al., reported that plantar desquamation offered a positive predictive value of 81% for presence of fungi. [29] However, none of the studies professes complete reliance on clinical features alone for a diagnosis of OM. The cost of treatment for OM is much more than the cost of laboratory testing. [27] Hence, laboratory diagnosis has been reported to be a must before starting therapy. The diagnosis of NDM OM may be tricky. Gupta et al., proposed that the diagnosis of NDM OM should be based on the presence of at least three of the following six criteria. These include identification of NDM in nail by direct microscopy; isolation of NDM in culture; repeated isolation in culture; inoculums counting; failure to isolate a dermatophyte in culture; and histology. [5] The method chosen by any practicing dermatologist depends on its sensitivity, specificity and availability. [Figure - 2] provides a detailed flowchart suggesting the diagnostic approach towards a case of OM.
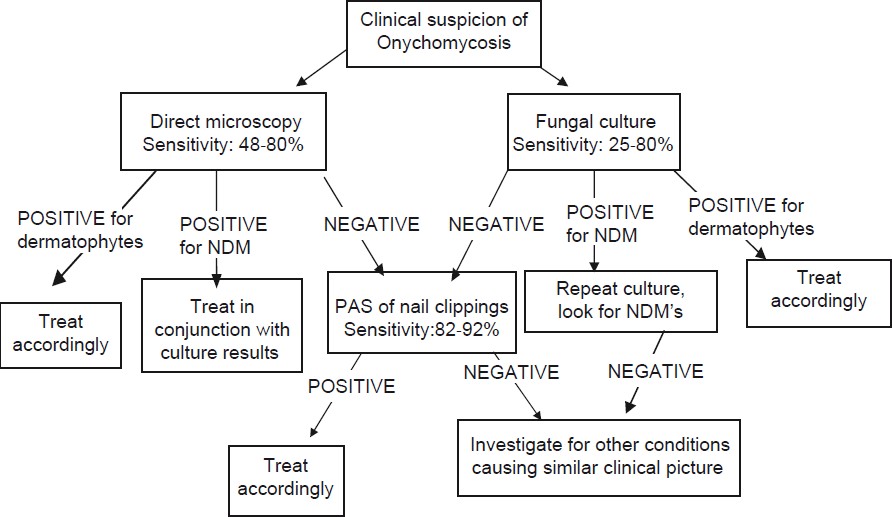 |
| Figure 2: A simplified algorithm for the diagnosis of OM |
Sampling techniques
Isolation of the fungal pathogen from nail is reportedly more difficult than for skin and hair. Conventionally, the material is taken from the distal end of the nail, despite the fact that the infection is advancing proximally. The hyphae at the distal end of the nail are less likely to be viable, hence less likely to grow on culture media. [30] Hence, culture results improve if samples are taken from more proximal sites. Proximal sampling may be more uncomfortable for the patients but it should be the norm as far as possible.
Detailed accounts of the techniques to sample nail unit are outside the scope of the present article. However, a few general tips which can improve the diagnostic yield are being offered. Nail clippers should be used to include full thickness of the nail. Subungual debris is a good source with superficial scrapings being helpful only in cases of SWO. Culture sensitivity from samples collected by drilling techniques has been found to be statistically superior to those collected by curettage. [31],[32] Drilling technique can be horizontal or vertical (especially useful for PSOM). [31] In an Indian study, the use of dental drill for collection of nail samples significantly improved the culture yield for fungus. [13]
Conventional methods of identifying causative organisms
A detailed review of the conventional diagnostic options is outside the scope of the present article. However, a brief but highly relevant account with newer suggested modifications is being offered for the sake of completion.
Direct microscopy
This is a rapid, simple and inexpensive technique to confirm the diagnosis in a clinical setting. The collected sample is incubated in 10-30% potassium hydroxide solution (KOH) so as to digest keratin, revealing the fungal hyphae. The higher the percentage of KOH, the faster is the clearing. Nail specimens take a longer time to clear than skin. If there is only subungual debris or very small pieces, specimen can be examined within 10 minutes with 10-15% KOH. However, if larger nail plate pieces are taken, they take a considerable longer time. [30] For them, the samples should be broken up into smaller parts initially itself and then incubated at 37 degree Celsius, for one minute and then examined.
Fungal elements are easily visualized at ×400 magnification on normal bright-field microscopy. Innovations in this technique include the use of phase contrast microscopy to differentiate between types of hyphae or arthroconidia; dark-field microscopy; use of calcofluor white or other special stains which help refine the process further. [33] Lim and Lim recently reported the use of CSB (Chicago sky blue) stain to improve the sensitivity as well as specificity of examination of suspected cases of onychomycosis with direct microscopy. [33]
Direct microscopy cannot differentiate between species but can give a clue to the possible group of fungi. In expert hands, a positive KOH with clinical suspicion of OM is adequate evidence for diagnosis. A sensitivity of 48% has been ascribed to KOH examination in a large study involving 631 nail samples. [34] Other authors have reported variable sensitivities between 50%-60% [35],[36],[37] while occasional studies have reported higher sensitivities approaching 80%. [38],[39]
Fungal culture
Culture was earlier considered the gold standard of diagnosis, being the only routinely available test which can identify the involved fungus. Reported sensitivity for culture varies from 25 to 80%. [37] Up to 30% cases may have false negative results especially when the sample is insufficient, taken from distal portions or is not crushed prior to inoculation. [37],[38]
Specimens should be plated on two different media; a simple medium like Sabouraud′s dextrose agar, which allows all fungi including yeasts and NDM to grow and a selective medium which contains ingredients like cycloheximide preventing the growth of saprophytes. [30] Cultures are incubated for three to four weeks and examined weekly. Fungal colonies are judged on the basis of growth patterns, color and microscopic formation of macro and microconidia or other typical growth features. [40] If growth is seen on both types of media, the infective agent is probably a dermatophyte, whereas growth only on the cycloheximide-free medium indicates that the infective agent may be an NDM. [41] Additional special culture media such as Potato Glucose Agar or Urea Agar may be needed to definitively differentiate between dermatophyte species. [40]
Diagnosis of NDM requires more than one laboratory analysis to show consistency of fungal growth. The criteria established by English et al., state that if a dermatophyte is isolated, it is considered to be the pathogen. [42] However, if moulds or yeasts are isolated, they are thought to be significant only if mycelia, arthrospores or yeast cells are found in the KOH test. Also, there should be isolation of at least five colonies (out of 20 nail fragments plated per person) of the same mould along with absence of dermatophytes, for the NDM to be considered a pathogen. [42] However, these criteria are stringent, difficult to fulfill and yield high false negative results. Hence, Shemer et al., [43] suggested that where NDM infection is present in the first culture, the patient should be re-examined on next visit and three separate samples should be taken from the affected nail. [43] If NDM is confirmed in all three of the cultures taken, the diagnosis of NDM is considered established and appropriate treatment can be started. [43]
Histopathology
Surgical pathology testing for OM using PAS (periodic acid Schiff stain) is the current Gold standard for the diagnosis of OM. [44] For histopathological examination, rather large nail clippings are taken, fixated for 4-8 hours in formalin and then softened [using agents like Potassium hydroxide (KOH), 5% trichloroacetic acid, 10% Tween- 40, or chitin softening solution containing mercuric chloride]. [41] Softened tissue sample are fixed in 10% buffered phosphate formalin for 24 h, dehydrated and then embedded. Semi-thin sections (5 microns) are taken using a microtome and stained with periodic acid-Schiff (PAS), the procedure altogether taking about 24-48 hours. [41] Nazarian et al., described the use of 20% NaOH pre-treatment of nail clippings for histopathology, prior to processing. [44] The study found that this creates significantly higher quality sections for both Hematoxylin and Eosin (H and E) stained and PAS-stained sections, with reduced tissue folding and fragmentation, improved ease of cutting and adherence of the tissue to glass slides. [44] Chang et al., studied 66 cases of onychomycosis wherein both the nail plate and subungual material were studied separately. Ninety-seven percent of these cases had hyphae in the subungual component. In 3% of cases, hyphae were present in the nail plate component but not in the subungual component. It was hypothesised that sufficiently sized subungual specimens are enough to confirm a diagnosis and even the nail plate may not be required. [41]
In a study by Wilsmann-Theis et al., PAS was found to be the most sensitive single test for the diagnosis for OM with a sensitivity of 82%, followed by culture (53%) and direct microscopy (48%). [34] They also evaluated 64 cases that were already on antimycotic treatment. Even in this subset, PAS was found to have the highest sensitivity (88%) in comparison with culture (33%) or direct microscopy (50%). [34] Histopathology not only proves the presence of fungus within the nail plate, but also gives a valuable clue to the level of invasion and its arrangement. Staining with Grocott methenamine-silver (GMS) has been shown to be qualitatively superior to PAS as it provides greater contrast between fungus and surroundings thus making identification easier. However, the procedure is more complex. [45]
Histopathological examination is useful for distinguishing between dermatophytes, yeast and NDM and allows for examination of mycelia threads and spore morphology in detail. [36],[41] However, the time required for the procedure is the disadvantage which needs to be overcome.
Newer methods
Polymerase chain reaction
Given the degree of uncertainty of conventional methods, various molecular biological techniques using PCR assay have been evaluated. They can provide a rapid, stable and accurate alternative for identifying pathogenic fungi both from the nail samples and from the fungal colonies. The methods used in samples from cultured colonies include arbitrarily primed PCR, PCR-restriction fragment length polymorphism (RFLP), double-round PCR, real-time PCR and PCR-direct sequencing. [46] In a study evaluating 550 affected nail samples, PCR has shown a sensitivity of 37% as compared to PAS (54%), KOH (40%) and culture (22%) respectively. [47] Specificity of PCR is excellent; however, there is an increased risk of contamination. Also, PCR cannot differentiate between pathogenic or nonpathogenic fungi. [48]
Li et al., developed and evaluated the efficacy of triplex PCR procedure to detect pathogenic fungi directly from specimens of OM. [48] They found the sensitivity of PCR, microscopy and culture to be 93.3%, 100% and 64.4%, respectively; specificities to be 100%, 86.4% and 100%, respectively; the positive predictive values to be 100%, 84.9% and 100%, respectively and the negative predictive values to be 95.2%, 100% and 78.7%, respectively. This molecular diagnostic process could distinguish the 3 groups of pathogens in onychomycosis (dermatophyte, yeast and mold) and could be completed within 8 h. [48]
Optical coherence tomography
It allows for noninvasive and noncontact cross-sectional imaging of biological tissue by detecting backscattering near infrared light of the inhomogenities within the sample. The longitudinal and transverse tomograms in OM show a thickening of the nail plate within which signal intense structures surrounded by low scattering areas are visible. [49] If histologically correlated, the high-scattering structures are conglomerates of hyphae which reflect more light due to their high chitin concentration and hence appear with higher signal intensity. The low scattering areas represent the surrounding lacunae of the hyperkeratotic nail plate. The results of OCT are comparable to the findings of PAS stained specimen and have been found to be superior to KOH preparations and cultures. [49] Thus, OCT is a reliable, easy to use, noninvasive and nondestructive method to visualize fungal elements in vivo, even in cases with false negative KOH-preparation and culture. Furthermore, it offers the opportunity to screen several areas within a nail plate and hence detect persisting fungal elements during local or systemic therapy. [49] However, larger studies are required to confirm the utility of this procedure. Also, its availability and cost effectiveness are not favorable for use in clinical practice.
Confocal laser scan microscopy
This is an in vivo test described for use in diagnosis of OM. [50] No further studies have been carried out using this technique, as it is expensive and complicated and hence, unsuitable for routine use. Using this technique, differentiating between cell membrane and fungal invasion of keratin is not easy. It has been reported that misdiagnosis is common and the procedure can be time consuming. [51]
Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS)
This technique is based on the detection of biochemical characteristics which are a result of the activity of mycological infections or noninfectious diseases. These are represented by proteolytic degradation products of native nail proteins. [52] The technique analyses the protein patterns of nail samples by using small amounts of peptides derived from tryptic digests of collected samples. The peptide patterns of affected samples are identified by comparison with known peptide spectra from nail disorders stored in an already existing data base. [52] The technique does not require any living or nonliving fungal material to prove or to rule out OM. It is also able to discriminate between OM and nonfungal nail disorders offering a distinct advantage over the conventional methods of KOH and culture which only prove or rule out presence of fungi. [52] Observer skill is less important compared to other newer methods, as the results depend on software assisted analysis. The preparation of nail material is simple. The method is also fast, with results available within 24 hours.
Phase contrast hard X-ray microscopy
This technique uses phase contrast microscopes utilizing synchrotron radiation. [51] Synchrotron radiation can provide a precise image of an extremely small object because of its brightness and high spatial resolution (upto 70 nm). [51] Hence, it can precisely image the internal structure of dermatology specimens. This helps in observing minute structures without requiring fixatives or stains. [53] Resolution is also much improved. [51] The major advantage with this technique of microscopy is that, just like histopathology, it provides direct evidence of fungal invasion of nail plate, showing that the fungi are pathogenic.
Conclusions
To conclude, OM is an age old adversary. However, we are yet to understand its full pathogenesis. It offers a significant diagnostic challenge to the dermatologist as it is an infection at a sanctuary site and is associated with little or no inflammation. There is consensus on the fact that OM should be treated, however, diagnostic methods and techniques are still evolving and being refined. Treatment of OM is an area of active research; however, basic research into its pathogenesis and diagnosis is opening up entirely new avenues. In the years to come, we may be witnessing a whole new way of approaching OM.
| 1. |
Haneke E, Roseeuw D. The scope of onychomycosis: Epidemiology and clinical features. Int J Dermatol 1999;38:7-12.
[Google Scholar]
|
| 2. |
Haneke E. Fungal infections of the nail. Semin Dermatol 1991;10:41-53.
[Google Scholar]
|
| 3. |
Sobhanadri C, Rao DT, Babu KS. Clinical and mycological study of superficial fungal infections at Government General Hospital: Guntur and their response to treatment with Hamycin, Dermostatin and Dermamycin. Indian J Dermatol Venereol Leprol 1970;36:209-14.
[Google Scholar]
|
| 4. |
Karmakar S, Kalla G, Joshi KR, Karmakar S. Dermatophytosis in a desert district of Western Rajasthan. Indian J Dermatol Venereal Leprol 1995;61:280-3.
[Google Scholar]
|
| 5. |
Gupta AK, Drummond-Main C, Cooper EA, Brintnell W, Piraccini BM, Tosti A. Systematic review of nondermatophyte mold onychomycosis: Diagnosis, clinical types, epidemiology, and treatment. J Am Acad Dermatol 2012;66:494-502.
[Google Scholar]
|
| 6. |
Monod M, Capoccia S, Lechenne B, Zaugg C, Holdom M, Jousson O. Secreted proteases from pathogenic fungi. Int J Med Microbiol 2002;292:405-19.
[Google Scholar]
|
| 7. |
Hellgren L, Vincent J. Lipolytic activity of some dermatophytes. II. Isolation and characterisation of the lipase of Epidermophytonfloccosum. J Med Microbiol 1981;14:347-50.
[Google Scholar]
|
| 8. |
Ito T, Meyer KC, Ito N, Paus R. Immune privilege and the skin. Curr Dir Autoimmun 2008;10:27-52.
[Google Scholar]
|
| 9. |
Mathison GE. The microbiological decomposition of keratin. Ann Soc Belg Med Trop 1964;44:767-9.
[Google Scholar]
|
| 10. |
Dorschner RA, Lopez-Garcia B, Massie J, Kim C, Gallo RL. Innate immune defense of the nail unit by antimicrobial peptides. J Am Acad Dermatol 2004;50:343-8.
[Google Scholar]
|
| 11. |
Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. Activities of cathelin associated antimicrobial peptide of human neutrophils. Antimicrb Agents Chemother 1998;42:2206-14.
[Google Scholar]
|
| 12. |
Ito T, Ito N, Saathoff M, Stampachiacchiere B, Bettermann A, Bulfone-Paus S, et al. Immunology of the human nail apparatus: The nail matrix is a site of relative immune privilege. J Invest Dermatol 2005;125:1139-48.
[Google Scholar]
|
| 13. |
Kaur R, Kashyap B, Makkar R. Evaluation of clinicomycological aspects of onychomycosis. Indian J Dermatol 2008;53:174-8.
[Google Scholar]
|
| 14. |
Rashid A, Scott E, Richardson MD. Early events in the invasion of the human nail plate by Trichophytonmentagrophytes. Br J Dermatol 1995;133:932-40.
[Google Scholar]
|
| 15. |
Zais N. Superficial white onychomycosis. Sabouraudia 1966;5:99-103.
[Google Scholar]
|
| 16. |
Ravhitscheck F, Evron R. Invasion of nails in vitro by certain dermatophytes. J Invest Dermatol 1957;28:261-8.
[Google Scholar]
|
| 17. |
Yu RJ, Harmon SR, Grappel SF, Blank F. Two cell bound keratinases of Trichophytonmentagrophytes. J Invest Dermatol 1971;56:27-32.
[Google Scholar]
|
| 18. |
Zaias N. Onychomycosis. Arch Dermatol 1972;105:263-74.
[Google Scholar]
|
| 19. |
Elewski BE. Large scale epidemiological study of the causal agents of onychomycosis: Mycological findings from the multicenter onychomycosis study of terbinafine. Arch Dermatol 1997;133:1317-8.
[Google Scholar]
|
| 20. |
Tosti A, Baran R, Piraccini BM, Fanti PA. "Endonyx" onychomycosis: A new modality of nail invasion by dermatophytes. Acta Derm Venereol 1999;79:52-3.
[Google Scholar]
|
| 21. |
Elewski BE. Onychomycosis: Pathogenesis, diagnosis, and management. ClinMicrobiol Rev 1998;11:415-29.
[Google Scholar]
|
| 22. |
Cohen JL, Scher RK, Pappert AS. The nail and fungus infections. In: Elewski B, editor. Cutaneous fungal infections. New York, NY: Igaku-Shoin Inc.; 1992. p. 106-22.
[Google Scholar]
|
| 23. |
Zaias N, Glick B, Rebell G. Diagnosing and treating onychomycosis. J Fam Pract 1996;42:513-8.
[Google Scholar]
|
| 24. |
Dompmartin D, Dompmartin A, Deluol AM, Grosshans E, Coulaud JP. Onychomycosis and AIDS: Clinical and laboratory findings in 62 patients. Int J Dermatol 1990;29:337-9.
[Google Scholar]
|
| 25. |
Moreno-Coutiño G, Toussaint-Caire S, Arenas R. Clinical, mycological and histological aspects of white onychomycosis. Mycoses 2010;53:144-7.
[Google Scholar]
|
| 26. |
Baran R, McLoone N, Hay RJ. Could proximal white subungualonychomycosis be a complication of systemic spread? The lessons to be learned from Maladie Dermatophytique and other deep infections. Br J Dermatol 2005;153:1023-5.
[Google Scholar]
|
| 27. |
Arrese JE, Piérard-Franchimont C, Piérard GE. Facing up to the diagnostic uncertainty and management of Onychomycoses. Int J Dermatol 1999;38:1-6.
[Google Scholar]
|
| 28. |
Fletcher CL, Hay RJ, Smeeton NC. Onychomycosis: The development of a clinical diagnostic aid for toenail disease. Part I. Establishing discriminating historical and clinical features. Br J Dermatol 2004;150:701-5.
[Google Scholar]
|
| 29. |
Doval G, Cabo F, Monteagudo B, Alavarez J, Ginarte M, Rodriguez-Alvarez MX, et al. Clinical diagnosis of toenail onychomycosis is possible in some patients: Cross-sectional diagnostic study and development of a diagnostic rule. Br J Dermatol 2010;163:743-51.
[Google Scholar]
|
| 30. |
Hay RJ, Ashbee HR. Mycology. In: Burns T, Breathnach S, Griffiths C, Cox N, editors. Rook's Textbook of Dermatology. 8 th ed. UK: Wiley-BlackwellPublisher;2010. p. 36.1-36.93.
th ed. UK: Wiley-BlackwellPublisher;2010. p. 36.1-36.93.'>[Google Scholar]
|
| 31. |
Shemer A, Trau H, Davidovici B, Grunwald MH, Amichai B. Nail sampling in onychomycosis: Comparative study of curettage from three sites of the infected nail. J Dtsch Dermatol Ges 2007;5:108-11.
[Google Scholar]
|
| 32. |
Shemer A, Davidoci B, Grunwald MH, Trau H, Amichai B. Comparative study of nail sampling techniques in onychomycosis. J Dermatol 2009;36:410-4.
[Google Scholar]
|
| 33. |
Lim CS, Lim SL. New contrast stain for the rapid diagnosis of onychomycosis. Arch Dermatol 2011;147:981-2.
[Google Scholar]
|
| 34. |
Wilsmann-Theis D, Sareika F, Bieber T, Schmid-Wendtner MH, Wenzel J. New reasons for histopathological nail-clipping examination in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol 2011;25:235-7.
[Google Scholar]
|
| 35. |
Shenoy MM, Teerthanath S, Karnaker VK, Girisha BS, Krishna Prasad MS, Pinto J. Comparison of potassium hydroxide mount and mycological culture with histopathologic examination using periodic acid-Schiff staining of the nail clippings in the diagnosis of onychomycosis. Indian J Dermatol Venereol Leprol 2008;74:226-9.
[Google Scholar]
|
| 36. |
Gianni C, Morelli V, Cerri A, Greco C, Rossini P, Guiducci A, et al. Usefulness of histological examination for the diagnosis of onychomycosis. Dermatology 2001;202:283-8.
[Google Scholar]
|
| 37. |
Lawry MA, Haneke E, Strobeck K, Martin S, Zimmer B, Romano PS. Methods for diagnosing onychomycosis: A comparative study and review of the literature. Arch Dermatol 2000;136:1112-6.
[Google Scholar]
|
| 38. |
Weinberg JM, Koestenblatt EK, Tutrone W, Tishler HR, Najarian L. Comparison of diagnostic methods in evaluation of onychomycosis. J Am Acad Dermatol 2003;49:193-7.
[Google Scholar]
|
| 39. |
Karimzadegan-Nia M, Mir-Amin-Mohammadi A, Bouzari N, Firooz A. Comparison of direct smear, culture and histology for the diagnosis of onychomycosis. Australas J Dermatol 2007;48:18-21.
[Google Scholar]
|
| 40. |
Seebacher C, Brasch J, Abeck D, Cornely O, Effendy I, Ginter-Hanselmayer G, et al. Onychomycosis. J Dtsch Dermatol Ges 2007;5:61-6.
[Google Scholar]
|
| 41. |
Chang A, Wharton J, Tam S, Kovich OI, Kamino H. A modified approach to the histologic diagnosis of onychomycosis. J Am Acad Dermatol 2007;57:849-53.
[Google Scholar]
|
| 42. |
English MP. Nails and fungi. Br J Dermatol 1976;94:697-701.
[Google Scholar]
|
| 43. |
Shemer A, Davidovici B, Grunwald MH, TrauH, Amichai B. New criteria for the laboratory diagnosis of nondermatophytemoulds in Onychomycosis. Br J Dermatol 2009;160:37-9.
[Google Scholar]
|
| 44. |
Nazarian RM, Due B, Deshpande A, Duncan LM, Misdraji J. An improved method of surgical pathology testing for onychomycosis. J Am Acad Dermatol 2011.[In press]
[Google Scholar]
|
| 45. |
Reza Kermanshahi T, Rhatigan R. Comparison between PAS and GMS stains for the diagnosis of onychomycosis. J Cutan Pathol 2010;37:1041-4.
[Google Scholar]
|
| 46. |
Ebihara M, Makimura K, Sato K, Abe S, Tsuboi R. Molecular detection of dermatophytes and nondermatophytes in onychomycosis by nested polymerase chain reaction based on 28S ribosomal RNA gene sequences. Br J Dermatol 2009;161:1038-44.
[Google Scholar]
|
| 47. |
Litz CE, Cavagnolo RZ. Polymerase chain reaction in the diagnosis of onychomycosis: A large, single-institute study. Br J Dermatol 2010;163:511-4.
[Google Scholar]
|
| 48. |
Li XF, Tian W, Wang H, Chen H, Shen YN, Lv GX, et al. Direct detection and differentiation of causative fungi of onychomycosis by multiplex polymerase chain reaction-based assay. Eur J Dermatol 2011;21:37-42.
[Google Scholar]
|
| 49. |
Abuzahra F, Spöler F, Först M, Brans R, Erdmann S, Merk HF, et al. Pilot study: Optical coherence tomography as a non-invasive diagnostic perspective for real time visualization of onychomycosis. Mycoses 2010;53:334-9.
[Google Scholar]
|
| 50. |
Hongcharu W, Dwyer P, Gonzalez S, Anderson RR. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol 2000;42:214-6.
[Google Scholar]
|
| 51. |
Lee O, Ha S, Lee G, Kim J, Huang J, Jin K, et al. Phase contrast hard Xray microscopy using synchrotron radiation for the diagnosis of onychomycosis. Microsc Res Tech 2010;73:1110-4.
[Google Scholar]
|
| 52. |
Pföhler C, Hollemeyer K, Heinzle E, Altmeyer W, Graeber S, Müller CS, et al. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: A new tool in diagnostic investigation of nail disorders? Exp Dermatol 2009;18:880-2.
[Google Scholar]
|
| 53. |
Kim GB. X-ray imaging of various biological samples using a phase contrast hard X-ray microscope. Microsc Res Tech 2008;71:639-43.
[Google Scholar]
|
Fulltext Views
11,146
PDF downloads
3,948





