Translate this page into:
Oral lichenoid reactions to talcum powder: A usual report with unusual history
Correspondence Address:
Santosh Palla
Department of Oral Medicine and Radiology, Indira Gandhi Institute of Dental Sciences, MGMCRI Campus, Puducherry - 607 402
India
| How to cite this article: Palla S, Rangdhol V, Shekar V, Jahan A J. Oral lichenoid reactions to talcum powder: A usual report with unusual history. Indian J Dermatol Venereol Leprol 2018;84:347-349 |
Sir,
We report a case of oral lichenoid reaction in a 41-year-old male patient who reported to the Department of Oral Medicine and Radiology, Indira Gandhi Institute of Dental Sciences, Puducherry. He presented with a complaint of oral pigmentation associated with a burning sensation. The history obtained from him, revealed a unique application of talcum powder intraorally with the belief that it will whiten his teeth. He reported applying this for 2 weeks for a period of 10 minutes, before brushing his teeth and later washing it off with water. On examination, dimensionally dissimilar grayish-white lesions were seen bilaterally on the buccal mucosae and the attached gingiva of all teeth. The lesions had no clinically detectable pattern and surrounding mucosa was normal [Figure - 1]a and [Figure - 1]b. They were provisionally diagnosed as “lichenoid reactions,” whereas lichen planus, stomatitis venenata and discoid lupus erythematosus were considered as differential diagnoses. The lesions were biopsied, and the histopathology showed orthokeratosis, acanthosis in spinous layer, “finger-like” rete ridges, basal cell degeneration, and juxtaepithelial band of chronic inflammatory cells [Figure - 2]a and [Figure - 2]b, suggestive of lichen planus. The final diagnosis of “oral lichenoid lesions histopathologically only compatible with lichen planus” was considered (as per WHO criteria). A patch test (antigen kit – Systopic labs, Delhi) was done, which was found to be negative for standard antigens [Figure - 3]a and [Figure - 3]b. The manufacturer details on the talcum powder, revealed the following compounds – (1) titanium dioxide, (2) calcium carbonate, (3) magnesium stearate, (4) alumina, silica, (5) vinyl dimethicone polymer and fragrance mix. These compounds were obtained (as raw samples from the Department of Chemistry, Puducherry) and applied with a petroleum jelly (as per Goossens [1]) for another patch test. The results showed an erythema reaction (graded as + as per Wilkinson and colleagues) to titanium dioxide (1), magnesium stearate (3), and negative to other 3 ingredients after 48 hours of application [Figure - 3]c. The patient was counselled regarding the lesions and was advised cessation of intraoral usage of the powder. The lesions showed regression in 3 weeks [Figure - 4]. The patient was advised repeated open application tests (ROATs) but he did not consent to the procedure as the lesions had regressed completely.
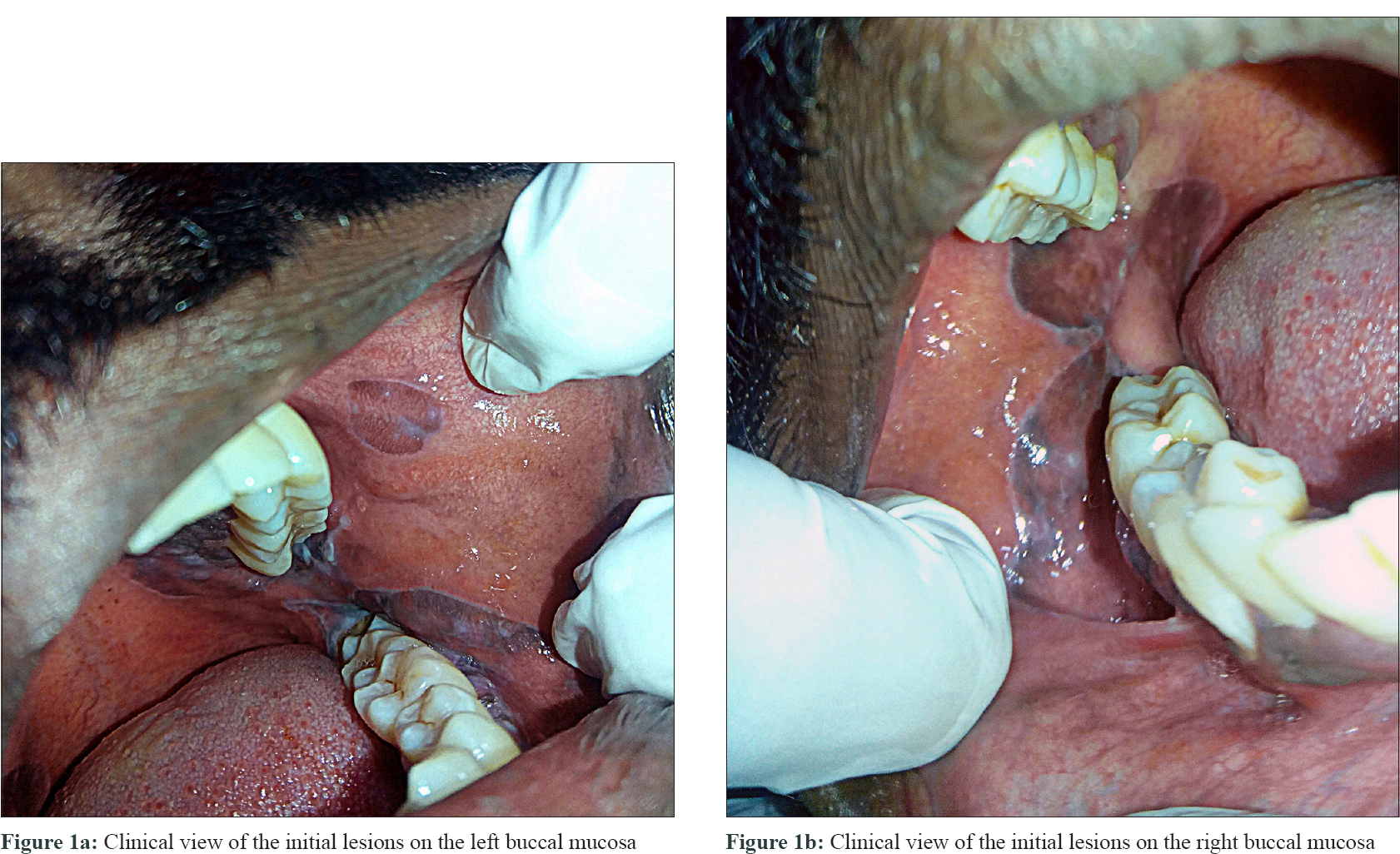 |
| Figure 1 |
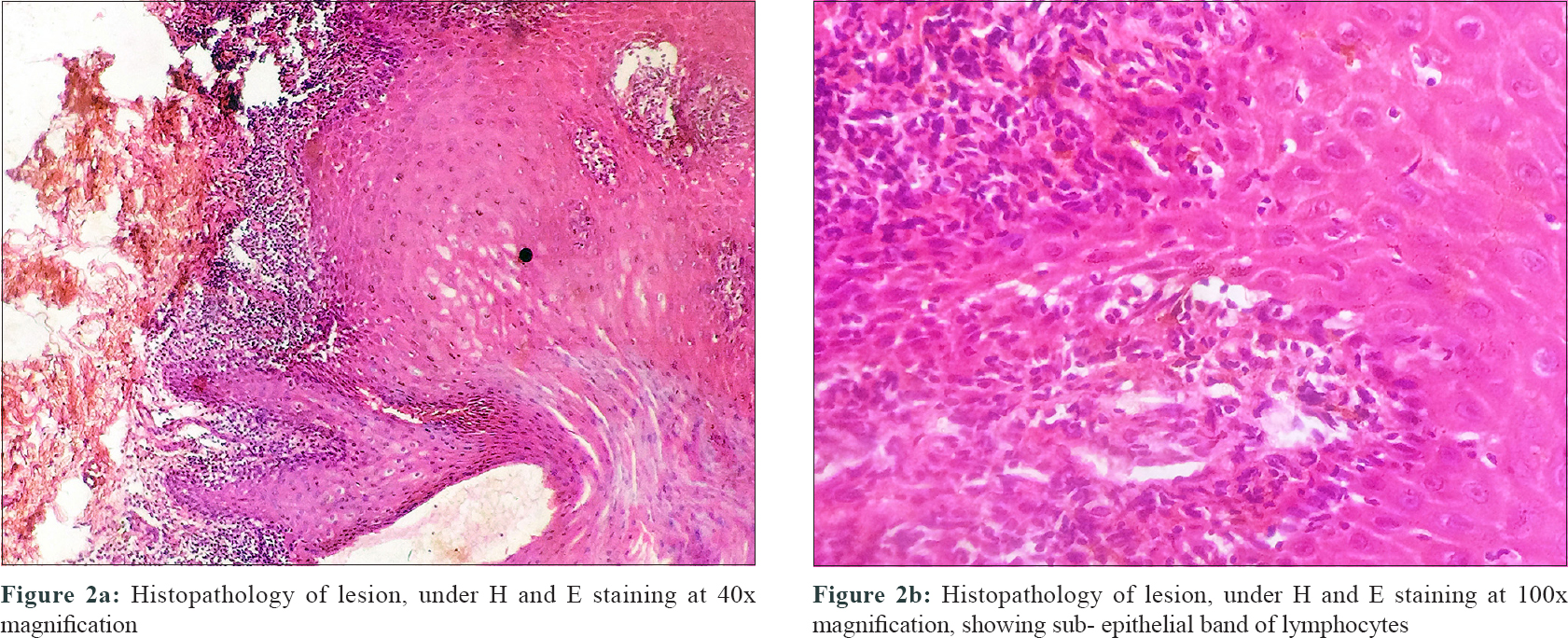 |
| Figure 2 |
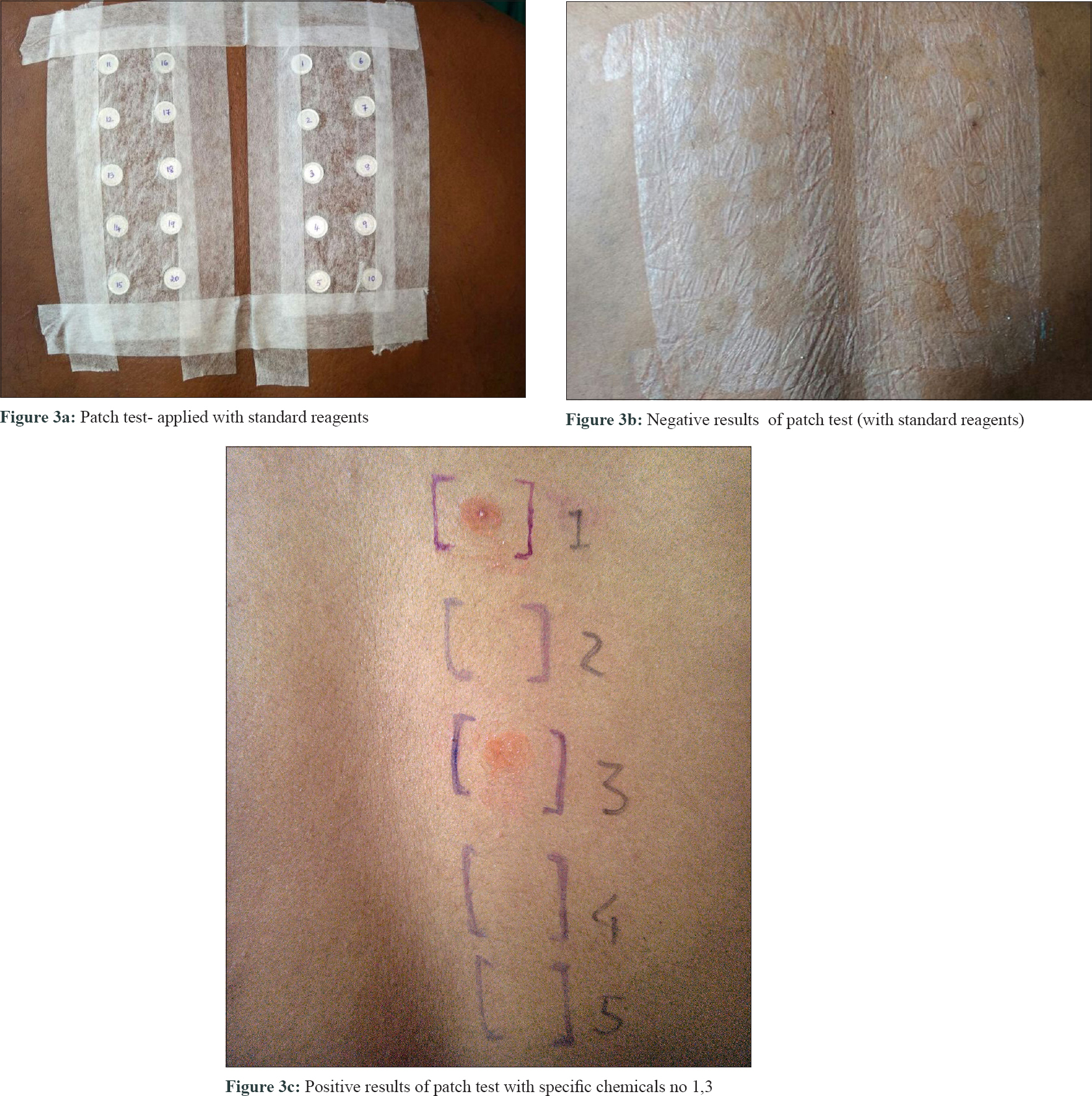 |
| Figure 3 |
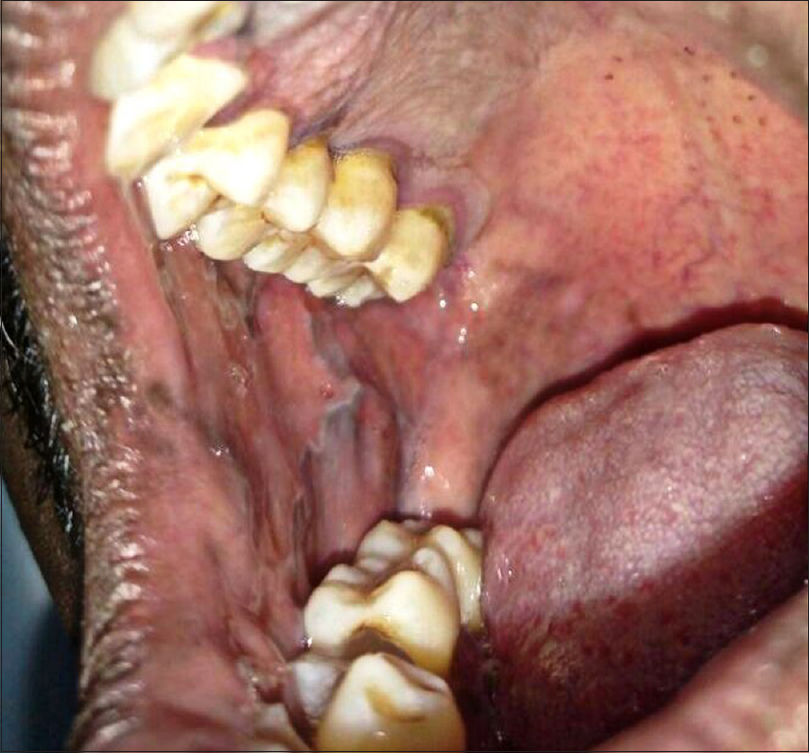 |
| Figure 4: Resolution of lesion (towards palate) and signs of regression (buccal mucosa) after discontinuation of irritant powder |
Oral lichenoid reaction is a clinical and histologic analogue of oral lichen planus (OLP), but has an associated cause and predicted outcome. The lichenoid lesions are examples of late reaction, manifesting similar to type IV hypersensitive reaction, lichen planus.
Literature search revealed that diagnostic criteria are not universal for oral lichenoid reactions and lichen planus.[2] WHO-modified classification is advised in reporting these lesions.[3] Drug interactions resulting in oral lesions clinically similar to lichen planus were first mentioned by Almeyda and Levantine (1971). Pinkus (1973) described that the oral mucosa or skin may exhibit similar clinical and microscopic alterations as that of lichen planus.[4] Currently, “oral lichenoid lesions” is the apt diagnostic term.
Oral reactions in our case were a consequence of lack of patient's knowledge regarding the chemical components of the talcum powder and the presumed false outcome of teeth whitening. The manufacturer's label clearly stated denial of application in any mucosal sites, but also mentioned a tag “lightening skin tone” which was misunderstood by the user. The association of an allergic compound triggering lichen planus and regression of the lesion without any intervention, can be used to differentiate oral lichenoid reactions from oral lichen planus.
The compounds implicated in patch testing, especially titanium dioxide, have to be considered as there is an evidence of reported damage to bronchial epithelium.[5] Clinicians can consider an association of mucosal lesions with a cosmetic containing titanium dioxide and magnesium stearate, as seen in this report. The association, however, needs to be established by further research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Goossens A. Art and science of patch testing. Indian J Dermatol Venereol Leprol 2007;73:289-91.
[Google Scholar]
|
| 2. |
Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: A systematic review. J Am Dent Assoc 2014;145:45-56.
[Google Scholar]
|
| 3. |
van der Meij EH, van der Waal I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med 2003;32:507-12.
[Google Scholar]
|
| 4. |
Almeyda J, Levantine A. Drug reactions. XVI. Lichenoid drug eruptions. Br J Dermatol 1971;85:604-7.
[Google Scholar]
|
| 5. |
Gurr JR, Wang AS, Chen CH, Jan KY. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005;213:66-73.
[Google Scholar]
|
Fulltext Views
2,568
PDF downloads
2,490





