Translate this page into:
Oxidative stress and leukocyte migration inhibition response in cutaneous adverse drug reactions
2 Department of Biochemistry, University College of Medical Sciences and Guru Teg Bahadur Hospital, University of Delhi, Delhi, India
3 Department of Pharmacology, University College of Medical Sciences and Guru Teg Bahadur Hospital, University of Delhi, Delhi, India
Correspondence Address:
Prashant Verma
University College of Medical Sciences and Guru Teg Bahadur Hospital, Shahdara, Delhi
India
| How to cite this article: Verma P, Bhattacharya SN, Banerjee BD, Khanna N. Oxidative stress and leukocyte migration inhibition response in cutaneous adverse drug reactions. Indian J Dermatol Venereol Leprol 2012;78:664 |
Abstract
Background: Cutaneous adverse drug reactions (CADRs) may either be immunological or non-immunological. The precise mechanisms, however, are largely obscure. Other concomitant mechanisms may amplify and/or contribute to the severity and duration of a reaction. One such mechanism could be oxidative stress, a state of imbalance between reactive oxygen species, and their subsequent detoxification by antioxidants. Aims: (a) to assess the oxidative stress status in the blood of cutaneous drug reaction patients by assaying for reduced glutathione (GSH) and malondialdehyde (MDA) levels, (b) to determine the leukocyte migration inhibition (LMI) response in these patients in response to the suspected drug (s), and (c) to look for the association between oxidative stress parameters and LMI. Methods: Ethical committee approval was obtained for this study. Fresh venous blood samples were obtained from the patients of CADRs (group A) during the acute phase of reaction and healthy control subjects (group B). MDA levels, a measure of oxidative lipid damage, and reduced GSH levels, a measure of anti-oxidant capacity, were assayed in the blood samples of both groups using spectrophotometry. LMI response was measured by challenging the patients' peripheral blood mononuclear cells with the suspected drug to confirm immunological perturbation. Results: Totally 66 participants, 33 cases in group A and equal number of controls in group B, were studied. The mean MDA levels were found to be raised (P < 0.001), but GSH levels were significantly reduced in group A when compared with group B (P = <0.001). LMI response against drug(s) was performed in 33 cases (group A), out of which 25 cases showed a positive LMI response as follows: fixed drug eruption (10/25), SJS (5/25), urticaria (3/25), exfoliative dermatitis (2/25), morbilliform rash (2/25), erythroderma (1/25), vasculitis (1/25), and dapsone syndrome (1/25). The mean MDA levels were found to be significantly higher in the LMI positive CADRs (P < 0.001) when compared with LMI-negative ones, while no significant difference was seen for GSH (P = 0.100). Furthermore, there was a significant positive correlation between MDA levels and LMI response (r = 0.831, P < 0.001). On the other hand, a negative but statistically insignificant correlation was found between GSH and LMI response (r = -0.248, P = 0.271). Conclusion: CADR patients were found to be under oxidative stress based on MDA and GSH levels in the peripheral blood. There is a significant positive correlation of LMI response (against the causative drug) with MDA levels, which strongly associates oxidative stress with the immunopathogenesis in CADRs.Introduction
Cutaneous adverse drug reactions (CADRs) constitute a repertoire of morphological reaction patterns that occur in response to a wide variety of drugs, each drug being capable of causing several different clinical types of reactions. It is difficult to pinpoint the pathological mechanism involved, on the basis of clinical appearances of a reaction alone. Therefore, the pathogenic mechanisms responsible for the various clinical types of adverse reactions, by any causative drug, are largely elusive.
CADRs may be immunological or non-immunological. [1],[2] A key step in the pathogenesis may involve release of leukocyte derived oxidants (reactive oxygen species or ROS). [3] A drug or its metabolite may haptenate endogenous proteins as carrier molecules forming an immunogen, which stimulates the immune system. [4] In this situation the stimulated leukocytes generate large amounts of ROS. [5] Further, some drugs are oxidants or can produce ROS directly. [6],[7],[8] These ROS can modify immune responses [9] (resulting in immunological tissue damage) and direct inflammatory tissue damage. [5] Leukocyte migration inhibition (LMI) in response to a drug exposure not only serves to assess and confirm the immunologic basis of a drug reaction but also can be used to identify the offending drug(s). [10]
Hence, the study of the state of oxidative stress in drug reactions can be used for further understanding of CADRs at molecular and cellular level, in addition to its diagnostic and therapeutic implications. In this direction, we deciphered to look for LMI response to drug(s) in cases of CADRs, in order to determine immune sensitization and to assay the oxidative stress parameters, and to analyze the correlation between these parameters.
Methods
This was a prospective study (conducted during the period 2004-2005) involving patients with a diagnosis of CADR. Thirty-three patients of both sex and all age groups with a diagnosis of CADR were enrolled. Oral drug provocation test was used where feasible i.e in non-severe reactions, exfoliative dermatitis, and vasculitis without systemic involvement. An equal number (n = 33) subjects, not having adverse reaction while taking the offending drugs (causally identified), constituted the control. Those patients with another disease that may cause oxidative stress such as tuberculosis, diabetes, and hypertension, and patients occupationally exposed to oxidants, were not included. Participants who had received systemic corticosteroids anytime after the onset of reaction, and pregnant and lactating mothers, were excluded. An informed consent was obtained from each patient. Based on the inclusion criteria, patients were divided into two groups-group A: patients (n = 33) with CADRs; group B: controls (n = 33), drug, age,-and sex-matched individuals.
Measurement of oxidative stress parameters
Estimation of lipid peroxidation (malondialdehyde): Malondialdehyde (MDA) levels were measured as an index of lipid peroxidation using the colorimetric method described by Satoh. [11] To 0.5 ml serum, 2.5 ml of trichloroacetic acid was added and the tube was kept for 10 min at room temperature. After centrifugation at 3500 rpm for 10 min, the supernatant was decanted and the precipitate was washed once with 2 ml 0.05 M sulphuric acid. Sulphuric acid (2.5 ml) and thiobarbituric acid (TBA) reagent (3 ml) were added to this precipitate and heated in a boiling water bath for 30 min to allow coupling of lipid peroxide with TBA. After cooling in cold water the resulting chromogen was extracted with 4 ml of n-butanol by vigorous shaking.
Separation of the organic phase was facilitated by centrifugation at 3500 rpm for 30 min and its absorbance was determined at wavelength of 532 nm by a spectrophotometer. The values were expressed in terms of concentration of MDA (nmol/ml).
Estimation of whole reduced glutathione (GSH) content: The GSH levels were determined by the method described by Matιs et al. [12] This method is based on the development of yellow color with 5,5΄-dithiobis-2- nitrobenzoic acid (DTNB), which is measured at 432 nm using a spectrophotometer. 0.2 ml of well-mixed anticoagulated venous blood was lysed with 1.8 ml of 1g/l EDTA. Three milliliter of precipitating reagent was added and after mixing the solution was allowed to stand for 5 min before being filtered through a single thickness Whatman no. 42 filter paper. Two milliliter of filtrate was then added to 4 ml of disodium hydrogen phosphate and 1 ml of DTNB reagent. A blank was prepared using 1.2 ml of precipitating reagent, 0.8 ml of EDTA solution and 4 ml of disodium hydrogen phosphate. One milliliter of DTNB reagent was added to both blank and test solutions. The color formed was then read in a spectrophotometer at 432 nm. The results were expressed in μmol/gHb.
LMI test: Leukocyte migration inhibition test (LMIT) against drug was carried out on peripheral blood mononuclear cells (PBMCs) of the subjects during clinical remission of a reaction. The method described by Banerjee and Hussain [13] was made use of for this purpose. Blood (4-5 ml) was withdrawn from each patient after clinical remission, and collected in 250 units of heparin and this was added to 2 ml of 3% dextran (molecular weight 200,000; BDH, UK) in 0.15 M NaCl and mixed well. The tube was kept in a slanting position for 1 h at 37°C and centrifuged at 400 g for 10 min at room temperature. The leukocyte pellet was washed twice with tissue culture media RPMI-1640, pH 7.2 and final suspension containing
15 × 0 6 leukocytes/ ml was prepared. Cell viability was determined by tryptan blue exclusion. Microcapillary tubes were filled with cell suspension and centrifuged at 250 g for 5 min after sealing one end with plasticine. The capillaries were cut at the cell pellet-medium interphase and the cell explants were mounted in Perspex chambers of 1.5 ml capacity with a dab of sterile silicon grease.
Tissue culture medium RPMI 1640 enriched with 5% new-born calf serum was used to fill the chambers. To one set of chambers, drug (in appropriate solvent) was incorporated in the medium and to another (control chambers) only medium and solvent were added. The chambers were closed with cover slips and incubated at 37°C in a humid atmosphere for 20 h. The areas of inhibition of leukocytes in control and test chambers were recorded on graph paper with the aid of a camera lucida. All tests were run in triplicate. The areas of migration of three duplicate capillaries were averaged and the percentage LMI was then calculated according to the following formula:
Percentage migration inhibition = 100 - area of migration with drug × 100
area of migration without drug
A percentage migration inhibition response of ≥20 was taken as positive.
Statistical analysis
Statistical analysis was done using unpaired t-test to compare the two groups (group A and group B) as well as LMI-positive and -negative subjects within group A for MDA and GSH. Furthermore, Pearson′s correlation was calculated to analyze the relation of LMI response with GSH and MDA in group A.
Results
Thirty-three adult patients diagnosed to have CADR on the basis of predetermined selection criteria, as outlined in the materials and methods, were subjects for the study. They were allotted into group A. Thirty-three age- and sex-matched healthy adult volunteers, who were given matched drugs and did not develop any reaction, formed the control group (Group B). The oxidative stress parameters were assayed as outlined in "Materials and Methods" for each subject of both the study groups (A and B). Blood sample was taken, during the acute phase of reaction. LMI was carried out for each subject of both the groups (A and B) after clinical remission. The values of each assayed parameter, in each of the study and control groups, were expressed as means ± standard deviation (M ± SD) and are shown in [Table - 1]. The data were analyzed using unpaired t-test for comparison.

Lipid peroxidation
MDA levels (nmol/ml) were measured in the serum, as outlined in "Materials and Methods" to estimate the presence and extent of oxidative damage to lipid membranes during the acute phase of a CADR and in controls.
The mean MDA level during the acute phase of CADR case group A was 6.735 ± 0.8843, while the mean MDA level of controls was 4.220 ± 1.5856.
A statistically significant difference was found between group A (case) and group B (control) (P < 0.001), indicating that MDA level in CADR cases is significantly raised when compared with the control group.
Glutathione redox system
Reduced GSH levels (μmol/Hb) were assayed in whole blood, as outlined in the Materials and Methods, as an indicator of the functional status of the GSH redox system, at inclusion, during the acute phase of CADR (group A) and once in control group (group B).
The mean GSH content of cases (group A) was 2.549 ± 0.3277 μmol/Hb and that of control (group B) was 4.466 ± 1.4466 μmol/Hb. A statistically significant difference was found between the groups, indicating that reduced GSH content in CADR cases is significantly reduced when compared with the control group (P = <0.001). The MDA values and reduced GSH levels, in CADR cases when compared with controls, are depicted in Table 1.
Leukocyte migration inhibition response
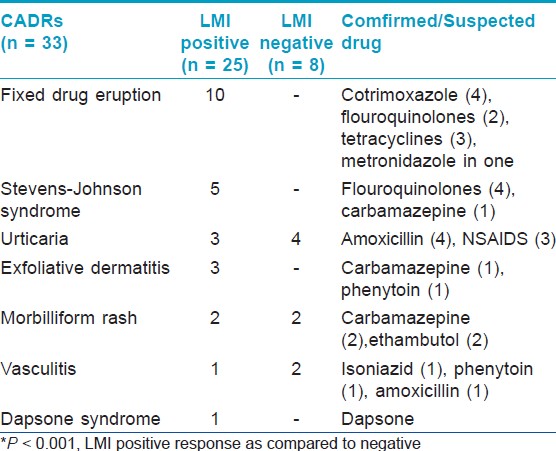
The LMI response to drug was measured in the PBMCs of the CADR cases, as outlined in the Materials and Methods, as an indicator of immunological mechanism of the CADR. LMI was positive in 25 of the 33 cases; FDE 10/10 (100%), SJS 5/5 (100%), urticaria 3/7 (42.8%), exfoliative dermatitis 3/3 (100%), morbilliform rash 2/4(50%), vasculitis 1/3 (33.33%), and dapsone syndrome 1/1 (100%). LMI response in group A (21.30 ± 7.502) was found to be significantly higher (P < 0.001) when compared with group B (2.523 ± 0.439). Of the 33 cases in group A, a positive LMI response was seen 25 subjects, while 8 were negative as depicted in [Table - 2]. The difference between mean LMI positive (25.58 ± 2.358) and mean LMI negative (9.89 ± 2.759) responses in CADR cases was found to be statistically significant (P < 0.001).
Correlation Between MDA and LMI Response and Between GSH and LMI Response
A significantly positive correlation was found between MDA and LMI response in group A (r = 0.831, P < 0.001), while a negative but statistically insignificant correlation was seen between GSH and LMI response (r = -0.248, P = 0.271).
Discussion
Cutaneous drug reactions are a repertoire of morphological reaction patterns, caused by a wide variety of drugs. The physiopathology of CADRs is largely obscure. They may be immunological or non-immunological; most of the reactions may be accounted for by the former. Immunological mechanisms are generally thought to revolve around T cell.
Under normal conditions, tissues are protected from the damaging effects of these ROS by antioxidant defense systems, which restore tissue homeostasis. When the generation of ROS alters the normal levels of these antioxidant systems, a state of oxidative stress is reached. [9] In this situation, several biological molecules involved in sensitive pathways such as cell signaling and gene regulation systems are altered. Many processes can modify the anti-oxidant status, including aging, nutrition, and drugs. [5],[9] Drug-associated effects have also been reported with GSH-peroxidase activity in patients taking paracetamol [6] and with superoxide dismutase activity in asthmatic patients taking corticosteroids. [7] Moreover, these same effects in the activity of these two enzymes have also been described at the same time in myocardium from a single experimental model after treatment with anti-hypertensive drugs. [8] These findings indicate that the drug itself may have some effect on the status of oxidative stress, probably because of their metabolic pathways. Furthermore, oxidative stress has been shown to modulate the expression of a variety of inflammatory molecules in several immunological diseases, such as atopic dermatitis, [14] psoriasis, [15] and asthma. [16] In this study, patients of Stevens-Johnson syndrome (SJS) with secondary infection were not excluded because that could have limited the number of cases to a very small number. Secondary infection in SJS could have affected the oxidative stress levels and might have confounded results to some extent.
Although specific immunological mechanisms are known to be involved in adverse drug reactions, [17] concomitant non-specific processes, such as oxidative stress, can also play a role, by amplifying the specific immune response. [18] Besides, oxidative stress may augment the metabolism of a drug leading to increased generation of active metabolites. [3],[4] Thus, these parameters were evaluated in the two groups, cases in acute phase of a CADR and controls consuming the drugs but not developing a reaction, and found a significant increase in MDA levels, while a significant decrease in GSH levels in CADRs when compared with controls. In the present work, the results are in consonance with previously published results. [18],[19],[20] The imbalance between oxidative damage and anti-oxidant capacity can lead to oxidative damage of proteins, as reflected especially by the high levels of MDA. Reduced GSH levels indicate the increased consumption of this antioxidant mechanism in quenching the free radicals. The negative correlation though not significant may signify that patients with immunological reactions have higher production of ROS which may deplete reduced GSH levels, an important antioxidant defence system.
As the study was focused on patients with immune -mediated cutaneous drug reactions, an attempt was made to determine whether there was a difference between LMI positive and negative CADRs with respect to MDA and GSH levels. The study was focused on PBMCs, because these are the cells most involved in immunological processes. LMI response is a simple and cheap method to identify immune sensitization. This method can identify immune sensitization in response to a drug with a sensitivity of 76%. [10] The study found that immune- mediated drug reactions, as determined by positive LMI response, had significantly raised MDA levels (marker of oxidative damage) as compared to non-immune reactions. Besides, there was a significant positive correlation between LMI response and MDA levels and a negative but insignificant correlation between LMI response and GSH. Although, an excessive production of ROS initiates oxidative stress and multiple pathological states are related to this situation, it is unclear whether this oxidative stress is the cause or the consequence of the immunological reaction to drugs. To conclude, further understanding of the mechanisms involved in oxidative stress as well as their relationship with the immunological response will aid the development of new therapeutic strategies in this and other related fields. In addition, oxidative stress parameters may form unique and valid markers of adverse drug reactions. LMI response is a simple and cost-effective in vitro method to determine immune sensitization of an individual to suspected drug(s), with a reasonable degree of sensitivity.
| 1. |
Breathnach SM. Drug reactions. In: Burns T, Breathnach SM, Cox N, Griffith C, editors. Rook's Textbook of Dermatology. 8 th ed. Oxford: Blackwell Scientific Publication; 2010. p. 75.
th ed. Oxford: Blackwell Scientific Publication; 2010. p. 75.'>[Google Scholar]
|
| 2. |
Sehgal VN, Jain S, Bhattacharya SN. Cutaneous drug reactions. J Eur Acad Dermatol 1993;2:281-95.
[Google Scholar]
|
| 3. |
Uetrecht JP. The role of leukocyte-generated reactive metabolites in the pathogenesis of idiosyncratic drug reactions. Drug Metab Rev 1992;24:299-366.
[Google Scholar]
|
| 4. |
Park BK, Kitteringham NR, Powell H, Pirmohamed M. Advances in molecular toxicology-towards understanding idiosyncratic drug toxicity. Toxicology 2000;153:39-60.
[Google Scholar]
|
| 5. |
Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol 2006;126:2565-75.
[Google Scholar]
|
| 6. |
Kozer E, Evans S, Barr J, Greenberg R, Soriano I, Bulkowstein M, et al. Glutathione, glutathione-dependent enzymes and antioxidant status in erythrocytes from children treated with high-dose paracetamol. Br J Clin Pharmacol 2003;55:234-40.
[Google Scholar]
|
| 7. |
De Raeve HR, Thunnissen FB, Kaneko FT, Guo FH, Lewis M, Kavuru MS, et al. Decreased Cu,Zn-SOD activity in asthmatic airway epithelium: Correction by inhaled corticosteroids in vivo. Am J Physiol 1997;272:148-54.
[Google Scholar]
|
| 8. |
Cabell KS, Ma L, Johnson P. Effects of antihypertensive drugs on rat tissue antioxidant enzymes activities and lipid peroxidation levels. Biochem Pharmacol 1997;46:133-41.
[Google Scholar]
|
| 9. |
Brigantiy S, Picards M. Antioxidant activity, lipid peroxidation and skin diseases. What's new. J Eur Acad Dermatol Venereol 2003;17:663-9.
[Google Scholar]
|
| 10. |
Uno K, Yamasaku F. Application of leucocyte migration tests to detection of allergenic drugs in patients with hypersensitivity to beta-lactam antibiotics. J Antimicrob Chemother 1989;24:241- 50.
[Google Scholar]
|
| 11. |
Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chem Acta 1978;90:37-43.
[Google Scholar]
|
| 12. |
Matés JM, Pérez-Gómez C, Olalla L, Segura JM, Blanca M. Allergy to drugs: Antioxidant enzymic activities, lipid peroxidation and protein oxidative damage in human blood. Cell Biochem Funct 2000;18:77-84.
[Google Scholar]
|
| 13. |
Banerjee BD, Hussain QZ. Effect of sub-chronic endosulfan exposure on humoral and cell-mediated immune responses in albino rats. Arch Toxicol 1986;59:279-84.
[Google Scholar]
|
| 14. |
Omata N, Tsukahara H, Ito S, Ohshima Y, Yasutomi M, Yamada A, et al. Increased oxidative stress in childhood atopic dermatitis. Life Sci 2001;69:223-8.
[Google Scholar]
|
| 15. |
Armstrong AW, Voyles SV, Armstrong EJ, Fuller EN, Rutledge JC. Angiogenesis and oxidative stress: Common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci 2011;63:1- 9.
[Google Scholar]
|
| 16. |
Nadeem A, Chhabre SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol 2003;111:72-8.
[Google Scholar]
|
| 17. |
Adkinson NF. Drug allergy. In: Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FE, editors. Middleton's allergy: Principles and practice. Vol. 2. Philadelphia: Mosby Inc.; 2003. p. 1679-94.
[Google Scholar]
|
| 18. |
Pérez-Gómez C, Segura JM, Blanca M, Asenjo M, Mates JM. Antioxidant activity levels and oxidative stress as blood markers of allergic response to drugs. Biochem Cell Biol 2000;78:691-8.
[Google Scholar]
|
| 19. |
Cornejo-Garcia JA, Mayorga C, Torres MJ, Fernandez TD, R-Pena R, Bravo I, et al. Anti-oxidant enzyme activities and expression and oxidative damage in patients with non-immediate reactions to drugs. Clin Exp Immunol 2006;145:287- 95.
[Google Scholar]
|
| 20. |
Tietz F. Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Application to mammalian blood and other tissues. Anal Biochem 1969;27:502-22.
[Google Scholar]
|
Fulltext Views
2,858
PDF downloads
1,633





