Translate this page into:
"Paradoxical" adverse effects caused by anti-tumor necrosis factor-alpha biological drugs: Appearance of psoriasis in a patient treated with infliximab for rheumatoid arthritis
2 Department of Clinical and Experimental Medicine, Second University of Naples, Naples, Italy
Correspondence Address:
Vincenzo Piccolo
Via Pansini 5, 80131 Naples, II Policlinico Ed. 3, 4� Piano
Italy
| How to cite this article: Satriano RA, Abbate G, Esposito S, Cassaglia B, Piccolo V, Baroni A. "Paradoxical" adverse effects caused by anti-tumor necrosis factor-alpha biological drugs: Appearance of psoriasis in a patient treated with infliximab for rheumatoid arthritis. Indian J Dermatol Venereol Leprol 2011;77:536 |
Sir,
Anti-tumor necrosis factor-alpha (TNF-α) drugs (both monoclonal antibodies such as adalimumab and infliximab and the soluble TNF-α-receptor such as etanercept) are indicated for the treatment of rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), Crohn′s disease (CD), and psoriasis. Their adverse effects also include the "paradoxical" psoriasis. [1],[2] We report a case of a "paradoxical" new-onset psoriasis in a 60-year-old woman, receiving infliximab. She was affected by RA since 14 months earlier and treated with methotrexate (15 mg/week). Due to the failure of treatment, we introduced infliximab (3 mg/kg every 8 weeks) with methotrexate (7.5 mg/week). After the fourth infusion of anti-TNF-α-drug, some erythematous squamous patches progressively appeared; they were discrete or coalescing and affected upper limbs, trunk [Figure - 1], and lower limbs [Figure - 2]. Scalp and nails were not involved. The patient had a personal and familial anamnesis negative for psoriasis; there was no history of recent infections and life-stress events. A biopsy specimen obtained from a patch of the trunk showed parakeratosis, slightly increased mitotic index, and dermal lymphocytic infiltration [Figure - 3]. Clinical and histopathologic findings supported the diagnosis of psoriasis. Serologic tests showed a 1:320 titre of anti-nuclear antibodies (ANA), and a positive result for anti-native DNA antibodies, that were negative before starting treatment with infliximab. Treatment with infliximab was stopped, and psoriasis was successfully treated with topical steroids. To date (6 months), there has been no recurrence of RA, since the patient is treated only with methotrexate (at a dose of 15 mg/week).
 |
| Figure 1: Isolated and confluent erythematous– squamous patches located on the back, elbows, and buttocks |
 |
| Figure 2: (a) Plantar lesions characterized by the presence of an extensive scaling and crusting; (b) erythematous– squamous lesions predominantly located in periungueal areas |
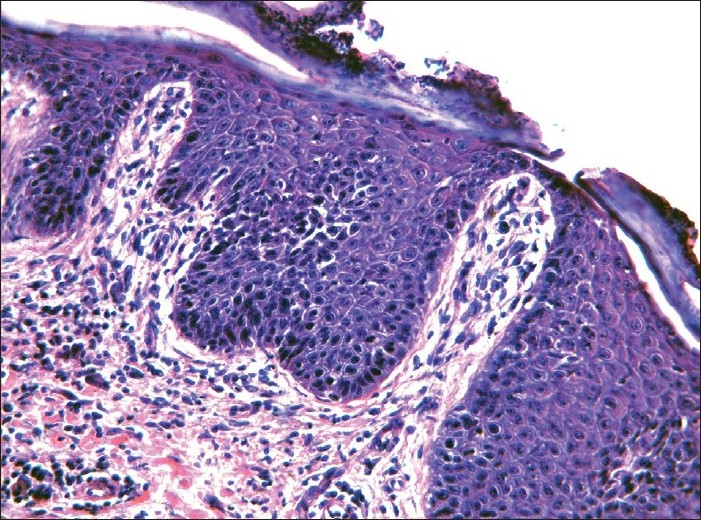 |
| Figure 3: Histopathologic examination is compatible with psoriasis due to the presence of parakaratosis, slightly increased mitotic index and dermal lymphocytic infiltrate (H and E, ×200) |
The new-onset psoriasis (or worsening of a pre-existing psoriasis) is increasingly reported as an adverse effect of treatment with anti-TNF-α drugs. [2] This event has been defined "paradoxical" since anti-TNF-α is indicated for the treatment of moderate-to-severe plaque psoriasis and could be considered a class effect of anti-TNF-α rather than a drug-specific adverse event. [2] The psoriasis "paradoxical" effect may occur whenever infliximab is indicated: RA, AS, CD, PsA, and psoriasis (in the last two conditions as worsening of a pre-existing disease). The risk of developing a psoriasis "de novo" is higher under anti-TNF-α antibodies treatment (with a slightly higher risk, in RA, for adalimumab compared to infliximab) [3] while the risk of worsening of a pre-existing psoriasis is higher by using etanercept. [2] The clinical expression is variable: it is usually a palmoplantar pustular psoriasis type; less frequently classic forms of psoriasis. Pathogenesis of this paradoxical effect is not well known, but the relationship between TNF-α and IFN-α has been emphasized. [2] TNF-α inhibits the maturation of plasmacytoid dendritic cells from hematopoietic progenitors and related production of IFN-α; thus blocking TNF-α causes an increased production of IFN-α, inducing psoriasis in some patients. An increased activity of IFN-α-type-I has often been found in the skin of patients treated with anti-TNF-α. [3] It has not been confirmed in skin lesions on a murine model, subject to TNF-α blockade; [3] the same authors hypothesized that an additional mechanism, found on a murine model, could explain the anti-TNF-α-induced psoriasis: these drugs are supposed to stimulate directly and/or indirectly the function of T-helper 17 (Th17) cells by decreasing the expansion of regulatory-T-cells (a phenomenon that stimulates Th17 cell expansion). Cytokines produced by Th17 cells have been assigned a role in the immunopathogenesis of psoriasis; in particular IL-22 that has a direct and indirect effect on keratinocytes, by stimulating them to release antimicrobial peptides. Their chemotactic function contributes indirectly to tissue damage in skin inflammation sites. [4] Risk factors for this paradoxical effect are not known. Positivity for ANA, occurring in our patient, has also been reported; [5] it has been established that the impact of anti-TNF-α on IFN-α may be responsible for the development of ANA, and of a lupus-like syndrome in patients undergoing treatment with anti-TNF-α. [3] Subacute lupus erythematosus may also present with psoriasiform lesions, but in our case it was ruled out due to histopathologic examination; therefore, the presence of a positive ANA titer has no clinical significance, it has to be considered only a not uncommon finding in patients undergoing anti-TNF-α. However, we do not know how much the positivity of ANA is predictive for a new-onset psoriasis. We reported this case in order to underline the importance of monitoring this "paradoxical" adverse effect, which is useful to understand its pathogenesis, risk factors, and clinical approach. In fact, despite the presence of suggestions for a practical approach to the problem, [3] it is still unclear which is the strategy to follow in terms of suspension or continuation of treatment with the anti-TNF-α drug (the same one used previously or another one) or with other biological drugs.
| 1. |
De Gannes GC, Ghoreishi M, Pope J, Russell A, Bell D, Adams S, et al. Psoriasis and pustular dermatitis triggered by TNF-alpha inhibitors in patients with rheumatologic conditions. Arch Dermatol 2007;143:223-31.
[Google Scholar]
|
| 2. |
Collamer AN, Guerrero KT, Henning JS, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: A literature review and potential mechanisms of action. Arthritis Rheum 2008;59:996-1001.
[Google Scholar]
|
| 3. |
Harrison MJ, Dixon WG, Watson KD, King Y, Groves R, Hyrich KL, et al. Rates of new-onset psoriasis in patients with rheumatoid arthritis receiving anti-tumour necrosis factor alpha therapy: Results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2009;68:209-15.
[Google Scholar]
|
| 4. |
Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci U S A 2005;102:3372-7.
[Google Scholar]
|
| 5. |
Ma HL, Napierata L, Stedman N, Benoit S, Collins M, Nickerson-Nutter C, et al. Tumor necrosis factor á blockade exacerbates murine psoriasis-like disease by enhancing Th17 function and decreasing expansion of Treg cells. Arthritis Rheum 2010;62:430-40.
[Google Scholar]
|
Fulltext Views
1,729
PDF downloads
2,561





