Translate this page into:
Paradoxical reactions induced by tumor necrosis factor-alpha antagonists: A literature review based on 46 cases
Correspondence Address:
Alexandra Zota
Department of Dermatology, Colentina Clinical Hospital, Bucharest
Romania
| How to cite this article: Olteanu R, Zota A. Paradoxical reactions induced by tumor necrosis factor-alpha antagonists: A literature review based on 46 cases. Indian J Dermatol Venereol Leprol 2016;82:7-12 |
Abstract
Anti-tumor necrosis factor (TNFα) agents have acquired a prominent place in the treatment options for inflammatory disorders. Among the side effects of these agents are the so-called paradoxical reactions which have increasingly been reported in recent years. A review of literature was carried out using Medline (PubMed) database from January 2010 to December 2014 to collect all published articles on cases of anti-TNFα-induced psoriasis and psoriatic arthritis. Published articles were identified, reviewed and the relevant data extracted. A total of 22 studies (46 patients) fulfilled the inclusion criteria and were selected for analysis. Of the 46 patients, 45 (97.8%) developed psoriasis and 1 (2.1%) psoriatic arthritis. The mean age of patients was 47 years; three (6.5%) patients had a past history of psoriasis. Infliximab caused cutaneous reactions in the most number, 26 (56.5%) cases. Thirty seven (80.4%). patients developed primary plaque-type psoriasis. Women accounted for 86.9% of patients. There was complete resolution of psoriasis in 12 (26%) patients despite differences in the therapeutic approach. Cessation of the incriminated drug led to resolution of cutaneous lesions in 5 (10.8%), switching to another TNFα antagonist led to resolution in 6 (13%) and one (2.1%) patient improved despite continuation of the drug. As for the lone case of psoriatic arthritis, drug withdrawal did not result in improvement; only switching to another anti-TNFα agent helped. Since our sample was small, it was not adequately powered to draw any firm conclusions. However, in this analysis, we found that paradoxical reactions occurred predominantly in adult women, there were only isolated cases with a personal history of psoriasis, infliximab was responsible for most cases of these reactions and the most prevalent form was plaque-type psoriasis. The decision whether to continue or discontinue the triggering anti-TNFα agent should be individualized as results are highly variable.Introduction
With more people receiving anti-tumor necrosis factor agents, there is a general concern about the “paradoxical reactions” and ways to manage them. Most authors manage these from their personal experience.
A paradoxical reaction is defined as one in which there is either onset or exacerbation of a condition (symptom/disease) following administration of a treatment which had proved efficacious for the very same condition.
The spectrum of paradoxical reactions due to use of anti-TNFα agents is quite vast and may be grouped into cutaneous (psoriasis and psoriasiform lesions), arthropathic (arthritis) and digestive (inflammatory bowel diseases). Amongst these paradoxical reactions, psoriasis has been the first and most reported. A review of the published literature was carried out to document this kind of reaction.
The causal relationship between TNFα blockade and development of psoriasis was indirectly demonstrated by an analysis of data from the British Society for Rheumatology Biologics Register which revealed 25 cases of new-onset psoriasis in 9826 rheumatoid arthritis patients treated with TNFα antagonists and none in 2880 rheumatoid arthritis patients treated with disease-modifying antirheumatic drugs.[1] The first paradoxical reaction, described in 2004, was infliximab-induced psoriasis in a patient with Crohn's disease.[2],[3] From then on, the majority of subsequent reports have occurred in rheumatology patients. The largest study available on this topic was published in 2010 and included details of all published cases of anti-TNFα-induced psoriasis reported up to August 2009.[4] The second largest review published in 2009, comprised 127 cases of TNF-induced psoriasis observed between 1990 and 2007.[5]
Methods
Search strategy
A review of the literature was carried out by using Medline (PubMed) database from January 2010 to December 2014 to collect all published articles on cases of anti-TNFα-induced psoriasis and psoriatic arthritis. To identify all relevant articles published in English (case series and reports, reviews, letters to the editor) on this topic, we used the following search key words: “psoriasis,” “psoriatic arthritis,” “anti-tumor necrosis factor-alpha” “infliximab,” “adalimumab,” “etanercept,” “golimumab,” “certolizumab,” “paradoxical reaction.” In addition, to make a distinction between psoriasis and psoriasiform reactions, we added the search terms: “psoriasis,” “histopathological findings,” “clinically confirmed,” “dermatologist,” “psoriasiform reaction.”
Data extraction
Studies were selected based on their titles and abstracts if they were available, and full articles retrieved for more detailed analysis. Theoretical review articles that did not include additional cases were excluded as were studies that did not provide information on psoriasis and psoriatic arthritis which developed due to any anti-TNFα agent available. Each study included was individually reviewed to identify data concerning age, gender, personal history of psoriasis, biological medication administered, cutaneous lesion type (histopathologically confirmed psoriasis or clinically confirmed psoriasis by a dermatologist, de novo psoriasis or reactivation of psoriasis), cutaneous biopsy results, underlying disease, therapeutic approaches and outcomes. Non-specific or unavailable information was designated as unknown or unstated data.
Results
The characteristics of the included study population are described in [Table - 1].
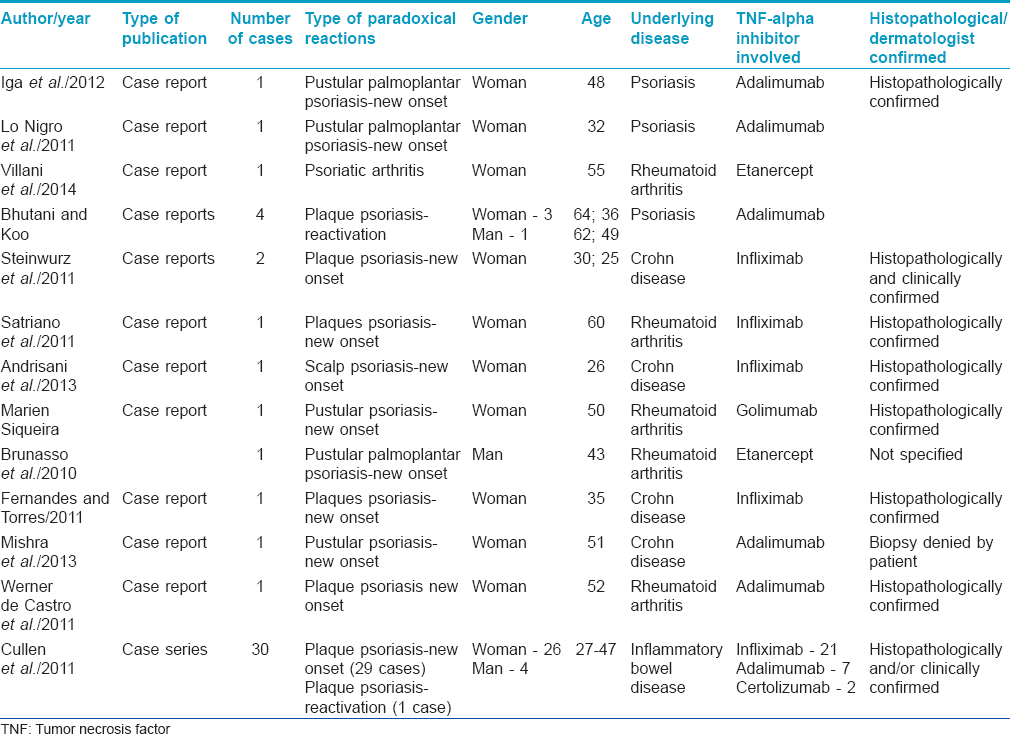
The reporting of various aspects of the cases was inconsistent across studies. For example, histopathological evaluation was not described in all cases.
During the study period, 22 publications were identified using the inclusion and exclusion criteria. A total of 46 patients experienced induction or exacerbation of psoriasis or psoriatic arthritis during anti-TNFα therapy as a paradoxical reaction.
Demographic aspects
There was a female preponderance, with a total of 40 (86.9%) females versus 6 (13.1%) males. Age ranged from 26 to 64 years with the mean age being 47 years. There were no patients under the age of 18 years.
There was no discernible demographic data to show predilection of this paradoxical reaction for any particular region or race.
Family history of psoriasis was negative in all patients while 3 (6.5%) had a personal history of the disease. No other potential precipitating causes for psoriasis were identified in any patient including major life stressors, trauma, or recent introduction of another medication.
Tumor necrosis factor-alpha inhibitors
Five anti-TNFα agents were responsible for induction or exacerbation of psoriasis/psoriatic arthritis (infliximab, adalimumab, etanercept, golimumab, certolizumab pegol) in the study population. Most cases were related to infliximab infusions, 26 (56.5%), followed by adalimumab in 15 (32.6%), etanercept in 2 (4.3%), golimumab in 1 (2.1%) and certolizumab pegol in 2 (4.3%) cases.
Clinical manifestations
The number of patients who developed paradoxical psoriasis, 45 (97.8%), clearly outnumbered the patients who developed psoriatic arthritis, 1 (2.1%).
Of the cases with psoriasis, 43 (93.4%) were new onset psoriasis while 2 (4.3%) had worsening of pre-existing psoriasis.
Clinical presentation varied; the most common psoriasis was plaque-type psoriasis in 37 (80.4%) cases followed by pustular in 5 (10.8%) and scalp psoriasis in 1 (2.1%) respectively.
The mean time of clinical latency between the beginning of biological therapy and the onset of psoriasis was 11 months; however, latency was up to a few years in same cases.
Histopathological evaluation
Most cases underwent a skin biopsy. Histopathological findings confirmed the diagnosis of psoriasis in 7 (15.2%) of the patients included in this review. The cases that were not submitted to cutaneous biopsy were clinically typical psoriasis and it seems likely that the diagnosis was accurate.
The underlying disease for which the patient received the anti-TNFα regimen was inflammatory bowel disease in 35 (76%) cases, the largest number, followed by rheumatoid arthritis in 5 (10.8%) and psoriasis in 6 (13%) cases.
Therapeutic approach
In most studies, complete remission of clinical lesions occurred with a wide range of therapeutic approaches. Anti-TNFα therapy was discontinued and adjuvant anti-psoriatic therapy introduced, resulting in complete response in 5 (10.8%) patients, a partial response in 1 (2.1%) and no change in 3 (6.5%) patients. In 1 (2.1%) patient, biological therapy was maintained resulting in improvement of cutaneous lesions. In 2 (4.3%) patients, systemic corticosteroids were administered. Regarding anti-psoriatic therapy, the vast majority of patients received topical corticosteroids with or without adjuvant therapy. Other therapeutic measures included acitretin, methotrexate, vitamin D3 and excimer light with variable results. In 6 (13%) patients, the anti-TNFα agent that triggered psoriatic lesions was replaced with another with resolution of lesions in all treated patients. In conclusion, cessation of the incriminated drug led to resolution of cutaneous lesions in 5 (10.8%) patients, switching to another tumor necrosis factor antagonist led to resolution in 6 (13%), and maintaining the drug led to resolution in 1 (2.1%) patient. As for psoriatic arthritis, drug withdrawal resulted in no improvement of the condition which was controlled only by switching to a different anti-TNFα agent.
Discussion
This study describes and analyses the clinical features and outcomes for a cohort of 46 reported cases of paradoxical reactions in the setting of anti-TNFα therapy between 2010 and 2014. The analysis serves as a useful summary of the currently available data.
Psoriasis and psoriatic arthritis, either new in onset, or with worsening of pre-existing lesions have been described in association with all presently licensed TNFα antagonists: infliximab, adalimumab, etanercept, golimumab and more recently certolizumab pegol. This paradoxical reaction has been described in rheumatologic conditions including rheumatoid arthritis and seronegative spondyloarthropathies, inflammatory bowel diseases and psoriasis.
Previously reported cases of these reactions have been described to occur at any age (mean age of 30.1 years) with no personal or family history of psoriasis in most patients (95%).[6] This review also found that these events were reported exclusively in adult patients who do not have a personal or family history of psoriasis.
Estimates of such cases in all patients treated with these agents are 5%, with minimal variations across studies.[7] This clearly exceeds the 1–2% prevalence expected by chance.
Although described cases in the literature have been reported to predominate in women, it is not possible to state that there is any gender bias.[6] In our review also, women account for 86.9% of total cases.
The pathogenesis of psoriatic skin lesions in the context of anti-TNFα therapy has received considerable attention. The most prominent hypothesis is that increased interferon-alpha associated with reduced circulating tumor necrosis factor leads to development of psoriatic lesions.[8] A genetic predisposition to these events is likely to exist as is an environmental triggering factor, given the highly variable timing of the reaction in relation to the initiation of anti-TNFα therapy.[8]
The most frequently described psoriasis in recent reviews is the pustular type (56%) which differs from the patients in this and other reviews, who primarily showed plaque-type psoriasis.[2],[3],[5],[6],[9],[10],[11],[12],[13]
Patients who have psoriasis lesions while undergoing anti-TNFα therapy should be referred to a dermatologist for a thorough evaluation. A biopsy should be performed with the aim of confirming psoriasis and to exclude other psoriasiform dermatoses. In this review, the clinical appearance of cutaneous lesions was highly suggestive of psoriasis in the majority of cases; 15.2% patients were biopsied, especially when the lesions were present in sites that were uncommon for psoriasis. Problems like eczema, atopic or seborrhoeic dermatitis were thus excluded by the authors. In addition, infections, mechanical stressors, trauma and medications which could trigger or exacerbate psoriasis were all excluded.
Infliximab was involved in the majority (54.5%) of cases but this probably reflects the fact that this drug has been in use longer than other anti-TNFα agents.
The clinical latency period between anti-TNFα agent administration and development of psoriasis is highly variable and could even take years. The average time taken is 9.5 months in patients with different underlying diseases.[4]
In addition to understanding the characteristics of this condition, it is important to know whether anti-TNFα-induced psoriasis is a class effect and whether switching to an alternative medication with a similar mechanism of action can be successful in ameliorating it, without recurrence.
In our study, in 13% of patients, the triggering agent was replaced by another similar drug which resulted in complete resolution of lesions in all cases. This variable response within individual patients reinforces the likely involvement of an environmental trigger. It has been hypothesized that individuals whose cutaneous disease recurs despite changing agents probably have an underlying predisposition to develop psoriasis while those whose skin findings resolve completely are more likely to have a true drug-induced psoriasis.[9]
Various therapeutic approaches have been described for treatment of psoriasis subsequent to anti-TNFα agent administration.[14],[15],[16] Regardless of the underlying disease, interruption or replacement of the drug culminated in the resolution of cutaneous lesions in 24% and 15% of cases, according to two recently published studies.[3]
To date, no guidelines exist for treating these reactions.[2] There is disagreement as to whether discontinuation of the incriminated drug is needed to achieve complete resolution of the lesions.[17],[18],[19] In our study, withdrawal of the involved agent resulted in complete resolution of cutaneous lesions in 5 (10.8%) cases while replacing it with another agent of the same class was successful in 13% of the analyzed cases. Nevertheless, further studies are still required to characterize better the efficacy and safety of this approach. However, discontinuation of anti-TNFα therapy should be particularly considered in generalized, recalcitrant cases and when the quality of life is severely impaired.[2]
The limitation of our article is that the analyzed sample size is small and therefore not adequately powered to draw any firm conclusion.
Conclusion
This case series analysis highlights an important unexpected and paradoxical event seen in association with use of TNFα antagonists, and the need for continued vigilance in all patients treated with these medications.[20],[21] A consensus regarding the therapeutic approach towards these reactions cannot be reached at this juncture, and customized management should be considered for each patient.
Financial support and sponsorship
Nil.
Confl icts of interest
There are no conflicts of interest.
| 1. |
Russell AS, Rosenbaum JT. Anti-tumor necrosis factor therapies in immune-mediated rheumatic diseases. Other observations from the clinic. J Rheumatol Suppl 2010;85:53-62.
[Google Scholar]
|
| 2. |
Denadai R, Teixeira FV, Steinwurz F, Romiti R, Saad-Hossne R. Induction or exacerbation of psoriatic lesions during anti-TNF-α therapy for inflammatory bowel disease: A systematic literature review based on 222 cases. J Crohns Colitis 2013;7:517-24.
[Google Scholar]
|
| 3. |
Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: Should biological therapy be suspended? Arq Gastroenterol 2012;49:172-6.
[Google Scholar]
|
| 4. |
Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: Clinical features and possible immunopathogenesis. Semin Arthritis Rheum 2010;40:233-40.
[Google Scholar]
|
| 5. |
Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: A review and analysis of 127 cases. J Dermatolog Treat 2009;20:100-8.
[Google Scholar]
|
| 6. |
Steinwurz F, Denadai R, Saad-Hossne R, Queiroz ML, Teixeira FV, Romiti R. Infliximab-induced psoriasis during therapy for Crohn's disease. J Crohns Colitis 2012;6:610-6.
[Google Scholar]
|
| 7. |
Brunasso AM, Laimer M, Massone C. Paradoxical reactions to targeted biological treatments: A way to treat and trigger? Acta Derm Venereol 2010;90:183-5.
[Google Scholar]
|
| 8. |
Lo Nigro A, Ramonda R, Alaibac M, Modesti V, Punzi L. Multiple paradoxical adverse events in a patient affected with ankylosing spondylitis treated with TNF blockers. Eur J Dermatol 2011;21:263-4.
[Google Scholar]
|
| 9. |
Cullen G, Kroshinsky D, Cheifetz AS, Korzenik JR. Psoriasis associated with anti-tumour necrosis factor therapy in inflammatory bowel disease: A new series and a review of 120 cases from the literature. Aliment Pharmacol Ther 2011;34:1318-27.
[Google Scholar]
|
| 10. |
Cleynen I, Vermeire S. Paradoxical inflammation induced by anti-TNF agents in patients with IBD. Nat Rev Gastroenterol Hepatol 2012;9:496-503.
[Google Scholar]
|
| 11. |
Sherlock ME, Walters T, Tabbers MM, Frost K, Zachos M, Muise A, et al. Infliximab-induced psoriasis and psoriasiform skin lesions in pediatric Crohn disease and a potential association with IL-23 receptor polymorphisms. J Pediatr Gastroenterol Nutr 2013;56:512-8.
[Google Scholar]
|
| 12. |
Soto Lopes MS, Trope BM, Rochedo Rodriguez MP, Grynszpan RL, Cuzzi T, Ramos-E-Silva M. Paradoxical reaction to golimumab: Tumor necrosis factor a inhibitor inducing psoriasis pustulosa. Case Rep Dermatol 2013;5:326-31.
[Google Scholar]
|
| 13. |
Satriano RA, Abbate G, Esposito S, Cassaglia B, Piccolo V, Baroni A. “Paradoxical” adverse effects caused by anti-tumor necrosis factor-α biological drugs: Appearance of psoriasis in a patient treated with infliximab for rheumatoid arthritis. Indian J Dermatol Venereol Leprol 2011;77:536.
[Google Scholar]
|
| 14. |
Mishra V, Daniel RC, Elmets CA, Levin A, Elewski BE. Palmoplantar pustulosis with fulminant dystrophic 20-nail psoriasis in a patient receiving adalimumab therapy. J Drugs Dermatol 2013;12:16-7.
[Google Scholar]
|
| 15. |
Fernandes IC, Torres T, Sanchez M, Velho G, Lago P, Selores M. Psoriasis induced by infliximab. Acta Med Port 2011;24:707-13.
[Google Scholar]
|
| 16. |
Werner de Castro GR, Neves FS, Pereira IA, Fialho SC, Ribeiro G, Zimmermann AF. Resolution of adalimumab-induced psoriasis after Vitamin D deficiency treatment. Rheumatol Int 2012;32:1313-6.
[Google Scholar]
|
| 17. |
Iga N, Otsuka A, Tanioka M, Miyachi Y, Kabashima K. Improvement of anti-TNF-α antibody-induced palmoplantar pustular psoriasis using a 308-nm excimer light. Case Rep Dermatol 2012;4:261-4.
[Google Scholar]
|
| 18. |
Andrisani G, Marzo M, Celleno L, Guidi L, Papa A, Gasbarrini A, et al. Development of psoriasis scalp with alopecia during treatment of Crohn's disease with infliximab and rapid response to both diseases to ustekinumab. Eur Rev Med Pharmacol Sci 2013;17:2831-6.
[Google Scholar]
|
| 19. |
Perez-Alvarez R, Pérez-de-Lis M, Ramos-Casals M. BIOGEAS Study Group. Biologics-induced autoimmune diseases. Curr Opin Rheumatol 2013;25:56-64.
[Google Scholar]
|
| 20. |
Villani AP, Weiler L, Jullien D, Chapurlat R, Confavreux C. Paradoxical psoriatic arthritis in a patient with rheumatoid arthritis treated by TNF-α blocker. Joint Bone Spine 2014;81:455-6.
[Google Scholar]
|
| 21. |
Bhutani T, Koo J. Paradoxical worsening of psoriasis when switching from etanercept to adalimumab: A case series. J Dermatolog Treat 2011;22:75-8.
[Google Scholar]
|
Fulltext Views
4,625
PDF downloads
3,443





