Translate this page into:
Paraneoplastic autoimmune multiorgan syndrome (paraneoplastic pemphigus) with unusual manifestations and without detectable autoantibodies
Correspondence Address:
Jimena Sanz-Bueno
Hospital 12 de Octubre, Avenida de C�rdoba s/n, Madrid - 28041
Spain
| How to cite this article: Sanz-Bueno J, Cullen D, Zarco C, Vanaclocha F. Paraneoplastic autoimmune multiorgan syndrome (paraneoplastic pemphigus) with unusual manifestations and without detectable autoantibodies. Indian J Dermatol Venereol Leprol 2014;80:328-330 |
Abstract
We describe a patient with paraneoplastic autoimmune multiorgan syndrome (PAMS) secondary to a lymphoblastic T- cell lymphoma who presented with a lichenoid dermatitis and vitiligo, later developing bronchiolitis obliterans and autoimmune hepatitis. Notably, he had no detectable autoantibodies. The development of vitiligo and autoimmune hepatic involvement probably indicate a role for cytotoxic T- cell lymphocytes in the pathogenesis of this syndrome.INTRODUCTION
Paraneoplastic pemphigus was first described by Anhalt et al., in 1990 [1] as an atypical pemphigus associated with lymphoid neoplasms and characterized by a vesiculobullous eruption mediated by circulating autoantibodies. [2] However, due to the significant phenotypic heterogeneity of the patients reported, some authors have proposed the term paraneoplastic autoimmune multiorgan syndrome (PAMS) to refer to this disease. [3] We report the case of a patient with PAMS secondary to a T-cell lymphoblastic lymphoma presenting with a graft-versus-host disease (GVHD)-like eruption and vitiligo, who developed lung and liver damage.
CASE REPORT
A 23-year-old man with right kidney congenital atresia of the right kidney and Barrett′s esophagus presented with fever, malaise, arthralgia, mouth erosions, and periocular erythematous patches in September 2008. Cutaneous lesions rapidly progressed along the craniocaudal axis affecting up to 90% of the body surface area. During the following months, they progressively acquired a lichenoid appearance. In March 2010, the biopsy of a cervical lymph node revealed a T-cell lymphoblastic lymphoma. Complete remission was achieved in August 2010 after completion of six cycles of R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone). Chemotherapy had no effect on skin disease which continued progressing. In October 2010, physical examination showed erythematous, scaly, papules and plaques with follicular plugging and a tendency to reticular pattern, palmoplantar involvement, nail pterygium, and loss of several nails. Diffuse alopecia and keratotic scales were noted on the scalp. Severe ulcers in the oral mucosa, conjunctivitis and erosions on the glans penis and secondary paraphimosis were present. Nikolsky′s sign was negative. Moreover, he developed poliosis of the eyebrows, the eyelashes, the hair and the beard, as well as multiple halo nevi [Figure - 1]. Progressive facial changes were noted, resulting in an elderly appearance [Figure - 2]. Several skin biopsies taken throughout the process showed hyperkeratosis with abundant necrotic keratinocytes in all layers of the epidermis, a band-like lympho-histiocytic infiltrate in the papillary dermis and vacuolar degeneration of the basal layer. No immune complex deposition or circulating autoantibodies were detected by direct or indirect immunofluorescence, respectively. Antibodies against periplakin, envoplakin, and both desmoglein 1 and 3 were negative. As high doses of oral steroids for long periods resulted only in a very slight improvement, cyclosporine was started at doses of 5 mg/kg/day. Two months later, the patient was admitted to the intensive care unit for fever and respiratory failure. Transbronchial biopsy revealed findings that were characteristic for bronchiolitis obliterans. Therapy with prednisone at doses of 1 mg/kg/day and monthly intravenous infusion of high-dose gamma globulin was required during the two months of hospitalization. In June 2011, he developed liver function impairment with a cytolytic pattern (aspartate aminotransferase (AST) and alanine aminotransferase (ALT) >1,000 UI/mL). Liver biopsy showed centrilobular necrosis with replacement of hepatocytes by lymphocytes and macrophages. These findings were strongly suggestive of autoimmune hepatitis, the resolution of which required high doses of prednisone (1 mg/kg/day) for one month. Currently, although no new signs of extracutaneous involvement are present and partial hair repigmentation and growth have been observed, an extensive lichenoid eruption involving almost the entire body persists, despite maintenance therapy with cyclosporine at doses of 3 mg/kg/day.
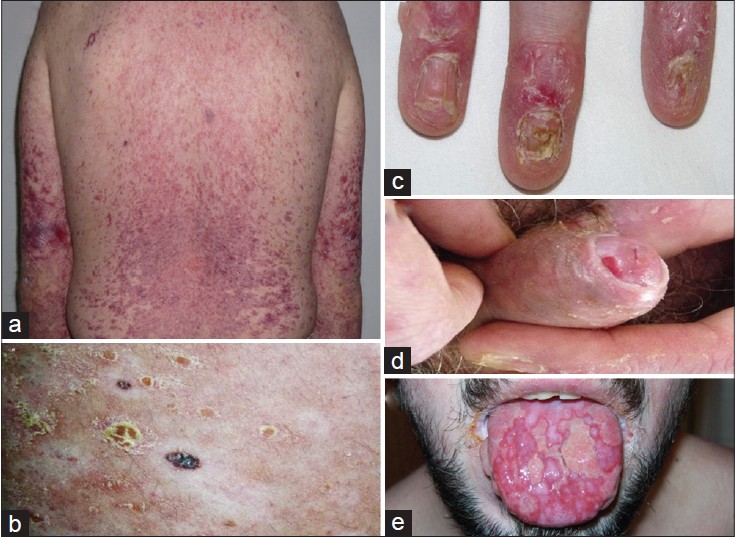 |
| Figure 1: (a) Erythematous-violaceous lichenoid papules over the trunk and the arms. (b) Scaly plaques, vitiligo, and halo nevi. (c) Onycholysis. (d) Erosions on the glans penis and secondary paraphimosis. (e) Ulcers on the tongue |
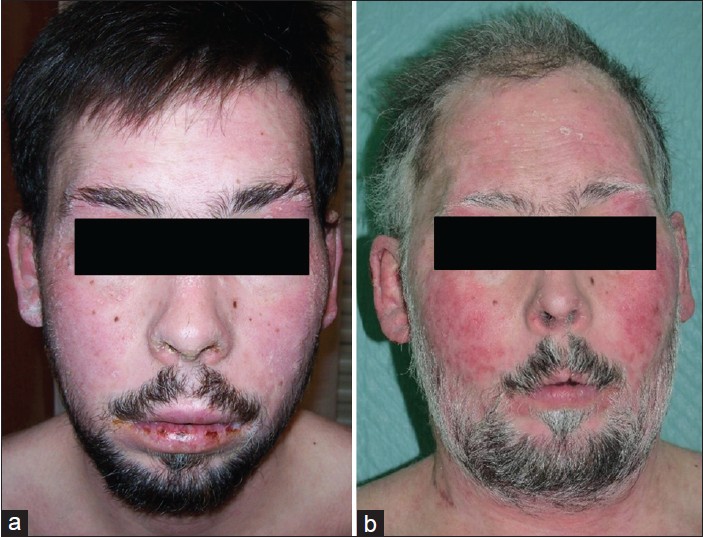 |
| Figure 2: (a) Seborrheic dermatitis-like patches and lip erosions in September 2008. (b) Facial erythematous plaques, poliosis, and elderly appearance in October 2010 |
DISCUSSION
Paraneoplastic autoimmune multiorgan syndrome is defined as a mucocutaneous eruption with a polymorphous presentation that has at least five clinical patterns (pemphigus, pemphigoid, exudative erythema multiforme, GVHD-like, and lichen planus-like), and that is accompanied by pulmonary involvement which is the main cause of death in these patients. [3] It is associated with several malignancies, of which hematologic-related neoplasms are the most frequent (84%) followed by adenocarcinomas and sarcomas. [4] Treatment of the underlying neoplasm is generally not associated with an improvement of the disease. [5] The most sensitive and specific antibodies in this disease are those targeting periplakin and envoplakin. [6] However, as was the case with our patient, Cummins et al., reported four cases of PAMS without autoantibodies. [7] This suggests that pathophysiological mechanisms are not only limited to humoral immunity. PAMS may represent a clinical spectrum in which predominance of humoral immunity would result in a bullous disease, as first described by Anhalt, and, conversely, the eruption may resemble GVHD or lichen planus when cell-mediated immune mechanisms are present, [8] as in this case. In fact, the development of vitiligo in our patient may support the theory of cell cytotoxicity. In contrast to bronchiolitis obliterans, autoimmune hepatitis has not been previously documented to be associated with PAMS. Our patient presented with unusual histological findings outside the context of a liver graft. This fact led us to think that, given that our patient had a healthy twin, and transplacental transfusions have been reported between dizygotic dichorionic twins, [9] a hypothetic lymphocyte population derived from his brother may had generated a GVHD-like reaction. However, the molecular analysis of the patient′s damaged skin and peripheral blood by polymerase chain reaction (PCR) failed to show mixed chimerism, rendering this possibility implausible. In sum, the aforementioned patient developed PAMS secondary to T-cell lymphoma with extensive mucocutaneous disease, vitiligo, elderly appearance, and autoimmune damage to the lung and liver. In spite of this dramatic clinical presentation and in contrast to most reports, he is still alive more than four 4 years after the diagnosis was made.
ACKNOWLEDGMENTS
We thank Dr. T. Hashimoto (Kurume University School of Medicine, Japan) for his help with immunofluorescence studies.
| 1. |
Anhalt GJ, Kim SC, Stanley JR, Korman NJ, Jabs DA, Kory M, Izumi H, Ratrie H 3 rd , Mutasim D, Ariss-Abdo L, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990 Dec 20; 323:1729-35.
[Google Scholar]
|
| 2. |
Anhalt GJ. Paraneoplastic pemphigus. Adv Dermatol. 1997; 12:77-96.
[Google Scholar]
|
| 3. |
Nguyen VT, Ndoye A, Bassler KD, Shultz LD, Shields MC, Ruben BS, Webber RJ, Pittelkow MR, Lynch PJ, Grando S et al. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: A reappraisal of paraneoplastic pemphigus. Arch Dermatol. 2001 Feb;137:193-206.
[Google Scholar]
|
| 4. |
Kaplan I, Hodak E, Ackerman L, Mimouni D, Anhalt GJ, Calderon S. Neoplasms associated with paraneoplastic pemphigus: A review with emphasis on non-hematologic malignancy and oral mucosal manifestations. Oral Oncol 2004; 40:553-562.
[Google Scholar]
|
| 5. |
Zhang J, Qiao QL, Chen XX, Liu P, Qiu JX, Zhao H, Zhao JX, Liu YC, Wan YL, et al. Improved outcomes after complete resection of underlying tumors for patients with paraneoplastic pemphigus: A single-center experience of 22 cases. J Cancer Res Clin Oncol. 2011 Feb; 137:229-34.
[Google Scholar]
|
| 6. |
Probst C, Schlumberger W, Stöcker W, Recke A, Schmidt E, Hashimoto T, Zhu XJ, Zillikens D, Komorowski L et al. Development of ELISA for the specific determination of autoantibodies against envoplakin and periplakin in paraneoplastic pemphigus. Clin Chim Acta 2009;410:13-18.
[Google Scholar]
|
| 7. |
Cummins DL, Mimouni D, Tzu J, Owens N, Anhalt GJ, Meyerle JH. Lichenoid paraneoplastic pemphigus in the absence of detectable antibodies. J Am Acad Dermatol 2007; 56:153-159.
[Google Scholar]
|
| 8. |
Czernik A, Camilleri M, Pittelkow MR, Grando SA. Paraneoplastic autoimmune multiorgan syndrome: 20 years after. Int J Dermatol. 2011;50:905-914.
[Google Scholar]
|
| 9. |
Jang JH, Jung H, Kim JH, Park WS, Kim SH. Blood chimerism in a dizygotic dichorionic pregnancy. Korean J Lab Med 2010;30:521-4.
[Google Scholar]
|
Fulltext Views
4,065
PDF downloads
2,620





