Translate this page into:
Paraneoplastic relapsing polychondritis and Sweet syndrome coexisting in a patient with myelodysplasia
2 Department of Dermatology and Venereology, Hospital S�o Jo�o, EPE, Porto; Faculty of Medicine (U38/FCT), University of Porto, Portugal
Correspondence Address:
Ana M Calistru
Department of Dermatology and Venereology, Hospital S�o Jo�o, EPE, Alameda Professor Hern�ni Monteiro, 4200-319, Porto
Portugal
| How to cite this article: Calistru AM, Lisboa C, Azevedo F. Paraneoplastic relapsing polychondritis and Sweet syndrome coexisting in a patient with myelodysplasia. Indian J Dermatol Venereol Leprol 2011;77:730 |
Sir,
Relapsing polychondritis (RP) is a rare inflammatory systemic disorder with cartilaginous tissues, such as ears, nose, joints and respiratory tract, as primary targets. Association of RP with hematological malignancies, particularly with myelodysplastic syndrome (MDS), has been described. [1] Sweet syndrome (SS) is an acute febrile neutrophilic dermatosis that may be related with neoplasm, especially hematological.
A 71-year-old man was referred to us for pain, edema and erythema of the left ear, sparing the ear lobe [Figure - 1]a and hand arthralgias, evolving for 3 weeks. He has been treated unsuccessfully with antibiotics for suspected infectious perichondritis. Two days prior to admission, the patient became febrile (38°C), developing erythematous infiltrated papules and plaques with pseudo-vesicles on his face, trunk and upper limbs [Figure - 2]a. There was no hearing loss, nor ocular or respiratory complaints. The patient was diagnosed with MDS 3 years before and was treated with blood transfusions and desferrioxamine. A biopsy of the ear lesion displayed a decreased basophilia of the cartilage, enlarged vacuolar spaces, marginal cartilage degeneration and adjacent inflammatory infiltrate [Figure - 1]b, consistent with chondritis. The trunk lesion histology revealed dermal edema, predominantly neutrophilic inflammatory infiltrate and negative direct immunofluorescence, compatible with SS [Figure - 2]b. The laboratory workup found pancytopenia, elevated C-reactive protein (42 mg/l; normal <3), a 1/100 titer of antinuclear antibodies (ANA) (normal<1/100) with homogeneous pattern, and an elevated titer of Anti-double stranded Deoxyribonucleic acid (anti-dsDNA) antibodies (31.7 UI/ml; normal<20) detected by Enzyme-linked immunosorbent assay (ELISA). The rheumatoid factor and the anti-extractable nuclear antigen antibodies were negative. The microbiologic examination of the ear fragment ruled out bacteriological, mycological and mycobacteriological infections. The head and neck computed tomography (CT)-scan showed no upper respiratory tract compromise. In addition, the immunophenotypic analysis of the peripheral blood and bone marrow examination did not indicate leukemia progression. Evidence of auricular chondritis with compatible histology features and inflammatory polyarthritis led to the diagnosis of RP, according to the Damiani and Levine criteria. [2] Concomitant SS diagnosis was achieved with two major criteria (sudden onset of specific lesions and consistent histopathology) and two minor criteria (associated hematological disorder and fever). The patient received Ibuprofen (400 mg, three times daily orally) and MDS treatment was optimized with the introduction of erythropoietin. Improvement of the RP symptoms was achieved after 2 weeks. The SS lesions resolved with potent topical steroid therapy (betamethasone dipropionate 0.05% cream, twice a day for 10 days). After a follow-up of 10 months, there was no record of relapse or new symptoms and the laboratory workup showed persistence of elevated anti-dsDNA (57 UI/ml) and borderline ANA antibodies (1/100).
 |
| Figure 1: (a) Edema and erythema of the left ear, sparing the ear lobe. (b) Histology of the ear fragment depicting a decreased basophilia of the cartilage, enlargement of the vacuolar spaces, marginal cartilage degeneration and adjacent inflammatory infiltrate (H and E, ×10) |
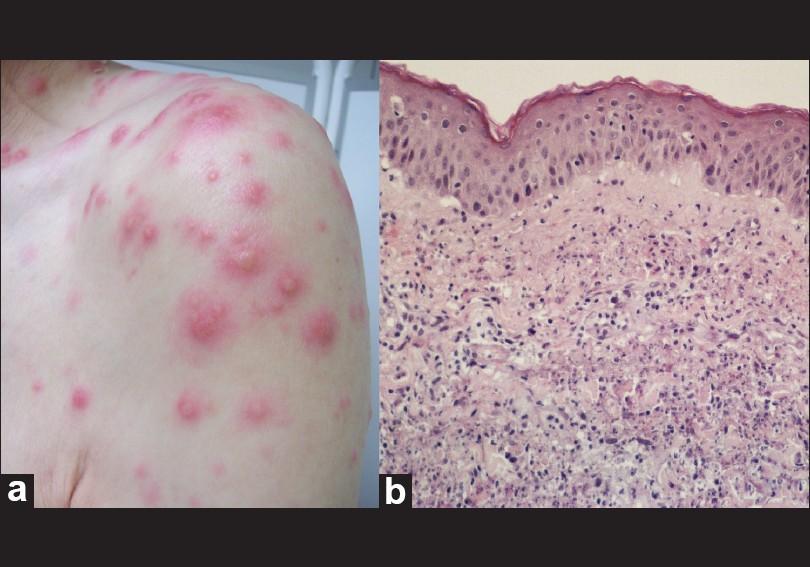 |
| Figure 2: (a) Infiltrated papules and plaques with pseudo-vesicles on the left shoulder. (b) Histological examination of a trunk lesion presenting dermal edema, predominantly neutrophilic inflammatory infiltrate (H and E, ×20) |
The etiology of RP is unknown, although there is evidence for autoimmune pathogenesis: the presence of T-cells infiltrate and antigen-antibody complexes in affected cartilages and circulating antibodies to cartilage-specific collagen types II, IX, XI. RP may occur in a primary form or in association with other disorders, such as autoimmune diseases and malignancies, where it is regarded as paraneoplastic.
Considering the present report, the association of RP and SS is documented in 23 cases. [1],[3],[4],[5],[6] Fourteen of these patients had a related malignancy: twelve had MDS, one had chronic lymphocytic leukaemia and another one had bladder cancer. Thus, it is conceivable that the development of SS and RP in the same patient is more than a mere coincidence, [1] implying a common cause or pathogenesis possibly related to neutrophil infiltration. [6] Immunological imbalances due to MDS concurrent with a specific immunogenetic background may play key roles in the pathogenesis of RP in these patients.
Autoimmune paraneoplastic syndromes or asymptomatic immunologic abnormalities may be encountered in patients with MDS. Our patient depicted an elevation of anti-dsDNA antibodies without fulfilling the criteria for systemic lupus erythematosus. The anti-dsDNA antibodies detected by ELISA may be present in up to 30% of other disease groups, such as rheumatoid arthritis, or in normal subjects. Accordingly, the elevated anti-dsDNA and the borderline ANA antibodies in our patient may represent either autoimmune manifestations related to RP or immunologic abnormalities associated to MDS. Systemic corticosteroids and non-steroidal anti-inflammatory drugs are the mainstay of RP therapy. [1] We decided to avoid systemic corticosteroid therapy due to the lack of multi-organ involvement and due to the additional risk of immunosuppression. Resolution of the symptoms was achieved through oral nonsteroidal anti-inflammatory treatment for chondritis and arthralgias, and with potent topical steroid for SS lesions. Herein, we report the association of two paraneoplastic syndromes with cutaneous expression in a patient with myelodysplasia. Our case also demonstrates the importance of recognizing RP in the differential diagnosis of an infectious perichondritis.
Further knowledge of the pathogenesis of RP, SS and MDS will provide important insights regarding the coexistence of these diseases.
| 1. |
Cohen PR. Sweet's syndrome and relapsing polychondritis: Is their appearance in the same patient a coincidental occurrence or a bona fide association of these conditions? Int J Dermatol 2004;43:772-7.
[Google Scholar]
|
| 2. |
Damiani JM, Levine HL. Relapsing polychondritis-report of ten cases. Laryngoscope 1979;89:929-46.
[Google Scholar]
|
| 3. |
Fraisse T, Brunel AS, Arnaud E, Balducchi JP, Jourdan J, de Wazières B, et al. Original presentation of relapsing polychondritis: Four cases. Rev Med Interne 2008;29:801-4.
[Google Scholar]
|
| 4. |
Kawakami T, Kawase A, Takeuchi S, Yoshioka S, Fujimoto N, Tajima S, et al. Sweet syndrome subsequent to relapsing polychondritis and myelodysplastic syndrome in a Japanese patient. Acta Derm Venereol 2008;88:517-9.
[Google Scholar]
|
| 5. |
Wastiaux H, Hervier B, Durant C, Gagey-Caron V, Masseau A, Barbarot S, et al. Sweet's syndrome as the presenting manifestation of relapsing polychondritis. Rev Med Interne 2010;31:e1-3.
[Google Scholar]
|
| 6. |
Vignon-Pennamen MD, Juillard C, Rybojad M, Wallach D, Daniel MT, Morel P, et al. Chronic recurrent lymphocytic Sweet syndrome as a predictive marker of myelodysplasia: A report of 9 cases. Arch Dermatol 2006;142:1170-6.
[Google Scholar]
|
Fulltext Views
3,109
PDF downloads
3,437





