Translate this page into:
Patch testing with expired Indian patch test kits: Results of a pilot study
Corresponding author: Dr. Vikram K. Mahajan, Department of Dermatology, Venereology and Leprosy, Dr. Rajendra Prasad, Government Medical College, Kangra (Tanda) - 176 001, Himachal Pradesh, India. vkm1@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mahajan VK, Chauhan PS, Mehta KS, Sharma A, Chowdhry B, Dhattarwal N, et al. Patch testing with expired Indian patch test kits: Results of a pilot study. Indian J Dermatol Venereol Leprol 2022;88:188-95.
Abstract
Background:
The reliability of patch testing with expired Indian standard patch test kits has been not evaluated before.
Methods:
Thirty adults (men:women 25:5) with allergic contact dermatitis were divided into three groups of ten patients each for patch testing by Finn chamber® method using Indian standard patch test kits having expiry in 2016, 2015 and 2014. The results were compared with those from a new kit with 2018 expiry.
Results:
Ten patients in group-1, eight patients in group-2 and seven patients in group-3 developed positive reactions of identical intensities and mostly from identical allergens from all four kits. The major contact allergens eliciting positive reactions of identical intensities were parthenium in nine, five and three patients, colophony in four, one and zero patients, fragrance mix in three, three and one patients, thiuram mix in three, one and one patients, and paraphenylene diamine in two, one and three patients from group-1,-2, and -3, respectively.
Limitations:
Small number of patients in each group remains the major limitation of the study. Whether or not these results can be extrapolated with patch test results from other similar patch test kits available across countries also needs confirmation.
Conclusion:
The patch test allergens can be used beyond labeled expiry dates but needs confirmation by a few large studies and using other available patch test kits. This is important as the relevance of patch test results for individual allergen in this scenario may remain debatable requiring careful interpretation.
Keywords
Airborne contact dermatitis
allergic contact dermatitis
Indian patch test series
parthenium
Patch test
Introduction
When performed accurately with correct interpretation of results, the patch test remains an important investigative tool to identify specific allergens responsible for causing allergic contact dermatitis, preventing recurrences and to differentiate allergic contact dermatitis from irritant contact dermatitis. Several allergens or haptens which are common sensitizers responsible for >80% of cases of allergic contact dermatitis are grouped together in a patch test battery or series. A patch test series usually includes metals (nickel, chromates, cobalt, etc.), rubber and leather chemicals, formaldehyde, lanolin, fragrances, constituents of cosmetics and toiletries, hair colorants, pharmaceutical items, preservatives and other additives from foods, beverages or other products of daily use, and extracts such as sesquiterpine oleoresin from Compositae plants (Parthenium hysterophorus in India) as common allergens. It is usually standardized for safety and reproducibility of results before approval/recommendation by national or international authorities/research groups prior to marketing and use in clinical practice. However, reluctance to do patch testing is not uncommon among dermatologists, who believe that it is not cost effective, is time consuming and needs several patient visits. Besides, unavailability of suitable test materials and the perceived risk involved in using a patch test kit beyond expiry date further discourage dermatologists from doing patch testing routinely. We studied the capacity of long-expired Indian standard patch test kits to elicit positive reactions in comparison to an un-expired kit.
Methods
The study comprised 30 patients aged 18 years and above and clinically suspected to have allergic contact dermatitis. After approval from Institutional Ethics Committee (Registration no ECR/490/Inst/HP/2013/RR-16) and informed/written consent, they were patch tested between Jan and Dec 2018 with Contact Dermatitis and Occupational Dermatoses Forum of India approved Indian standard patch test kit, marketed in India by Chemotechnique Diagnostics, Sweden, (www. chemotechnique.se) in collaboration with Systopic India Ltd, New Delhi (India) presently costing about rupees ten thousand each. Pregnant or lactating women were excluded from the study and patients having acute dermatitis were enrolled only after acute dermatitis had subsided and they were off systemic or topical corticosteroids for ≥2 weeks. All enrolled patients were hospitalized and subjected to detailed medical history and clinical examination and divided into three groups of ten patients each. The patch testing was performed by Finn chamber® method according to European Society of Contact Dermatitis (ESCD) guidelines with Indian standard patch test kits having expiry date in 2016, 2015 and 2014.1 The results were compared with those from a new patch test kit having expiry date in 2018 tested concurrently. All the patch test kits having identical allergens were purchased from Systopic India Ltd, New Delhi (India) and had been stored at 4ºC before testing. The Finn chambers (8mm) with test allergens were applied on non hairy upper back after gentle cleansing with ethyl alcohol and left for 48 h. The reading of results was done after 48 h (D2) and 72 h (D3).The results were graded according to the International Contact Dermatitis Research Group criteria.1 Only positive reactions persisting on D3 were considered positive and significant for final analysis. The relevance of positive patch test results was determined clinically as “definite” if the reaction was positive to the patch test allergen and exposure to it could be verified, “probable” if the presence of identified allergen in the known skin contactants could be verified, “possible” if the patient was exposed to the material known to have the putative allergen, “past” if a positive patch test reaction could be related to previous and unrelated episode of contact dermatitis and “unknown” if no relevance could be made out.2
Results
These 30 patients (men: women 25:5) were aged between 29 and 75 years (mean: 56.6 years). They were primarily involved in agriculture and related activities. They had allergic contact dermatitis for two months to 25 years (mean: 21.3 years) with exacerbations and remissions. Common clinical patterns were airborne contact dermatitis in eight patients, chronic actinic dermatitis in fourteen patients, facial, acral and acrofacial contact dermatitis in seven patients and scalp contact dermatitis in one patient.
Tables 1-3 depict detailed baseline characteristics and patch test results in each group. Intragroup comparisons showed that all the ten patients in group-1, eight patients in group-2 and seven patients in group-3 developed clinically relevant positive reactions of identical intensities from identical allergens from both the kits [Table 4]. The major contact allergens from both the patch kits eliciting positive reactions of identical intensities in group-1 were parthenium in nine patients, colophony in four patients, fragrance mix and thiuram mix in three patients each and paraphenylene diamine and black rubber mix in two patients each [Figures 1a and b]. The positive reactions due to other allergens such as chlorocresol, Myroxylon pereirae (balsam of Peru) and nitrofurazone were also similar from both the kits in a majority of the cases. Three men aged 63, 70 and 60 years (case-1,-2 and -3), patch tested 3, 4 and 5 years back had shown parthenium as a major contact allergen. Upon re-testing, they again showed positive reactions of the same intensity from both the kits. Similarly, among eight patients in group-2, parthenium in five patients and fragrance mix in three patients were the major allergens eliciting positive reactions of identical intensities [Figures 2a and b]. Positive reactions from neomycin, formaldehyde, potassium dichromate, epoxy resin, paraben mix, nitrofurazone, colophony, thiuram mix and wool alcohol were also of similar intensity from both the kits. In group-3 patients, paraphenylene diamine in three patients, parthenium in two patients and fragrance mix in one patient were the major contact allergens from both the kits eliciting positive reactions of identical intensities [Figures 3a and b]. Other common allergens with identical intensity of positive reactions included Myroxylon pereirae, potassium dichromate, paraben mix and chlorocresol in one patient each. One patient in group-3 developed angry back phenomenon from both the kits.
| Case number | Age in years/sex | Occupation | Sites of dermatitis | Clinical diagnosis | Duration | Patch test results (grades) ISPTS kit 2016* |
Patch test results (grades) ISPTS kit 2018* |
|---|---|---|---|---|---|---|---|
| 1 | 70/malea | Agriculture | Face, trunk and upper limbs | ABCD | 18 years | Parthenium (3+) | Parthenium (3+) |
| 2 | 60/maleb | Agriculture | Face, V area-neck and dorsal hands/feet | CAD | 10 years | Parthenium (3+) | Parthenium (3+) |
| 3 | 63/malec | Agriculture | Face, neck, back and limb flexures | ABCD | 20 years |
Parthenium (3+u) Fragrance mix (1+) |
Parthenium (3+u) Fragrance mix (1+) |
| 4 | 62/female | Homemaker/Agriculture | Face, hands and feet | Acrofacial dermatitis | 2 months |
PPD (3+), benzocaine (1+) and MBT (1+) |
PPD (2+), benzocaine (1+) and epoxy resin (2+) |
| 5 | 68/male | Agriculture | Face, neck and limb flexures | ABCD | 4 years |
Parthenium (3+) and colophony (1+) |
Parthenium (3+), colophony (1+) and Myroxylon pereirae(1+) |
| 6 | 70/male | Agriculture | Scalp, face, V area-neck and dorsal hands/feet | CAD | 2 years |
Parthenium (3+u), PPD (3+), colophony (3+), chlorocresol (3+) and thiuram mix (3+) Black rubber mix (3+), fragrance mix (2+), nitrofurazone (2+), Myroxylon pereirae (2+), paraben mix (1+), cobalt sulfate (1+) and formaldehyde (1+) |
Parthenium (3+u), PPD (3+), colophony (3+), chlorocresol (3+) and thiuram mix (3+) Black rubber mix (3+), fragrance mix (3+), nitrofurazone (1+), Myroxylon pereirae (2+) and paraben mix (1+) |
| 7 | 48/male | Self-employed (business), spare time agriculturist | Face, trunkand upper limbs | ABCD | 20 years |
Parthenium (3+), thiuram mix (3+), black rubber mix (1+), fragrance mix (1+) and nitrofurazone (1+) Formaldehyde (1+), neomycin (1+) and benzocaine (1+) |
Parthenium (3+), thiuram mix (1+), black rubber mix (1+), fragrance mix (1+) and nitrofurazone (1+) Formaldehyde (1+) and Myroxylon pereirae(1+) |
| 8 | 57/male | Office work (accountant) | Face, hands and feet | Acrofacial dermatitis | 15 years |
Parthenium (3+u), colophony (2+), thiuram mix (1+) and nitrofurazone (2+) |
Parthenium (3+), colophony (2+), thiuram mix (2+), MBT (2+) and wool alcohol (1+) |
| 9 | 46/male | Agriculture | Scalp, face, V area-neck and dorsal hands/feet | CAD | 7 years | Parthenium (1+) | Parthenium (1+) |
| 10 | 75/male | Agriculture | Face, neck and trunk upper limbs | ABCD | 4 years |
Parthenium (3+) Colophony (2+) and Chlorocresol (1+) |
Parthenium (2+) Colophony (2+) |
Positive results shown in bold indicate allergens common to both kits. “3+u” indicates intense positive reaction with ulceration. *All positive results were of definite clinical relevance, aPatch test results in 2013: parthenium (2+), colophony (1+), Myroxylon pereirae (1+) and nitrofurazone (2+), bPatch test results in 2014: parthenium (3+) and black rubber mix (2+), cPatch test results in 2015: parthenium (2+), fragrance mix (1+) and colophony (1+). Baseline characteristics: Males: Females 9:1; Age: 46-75 (mean 61.9) years; Duration of dermatitis: 2 months-18 (mean 10.02) years, Clinical patterns of dermatitis: ABCD in five patients, CAD in three patients and acrofacial dermatitis in two patients. ISPTS: Indian standard patch test series, MBT: mercaptobenzothiazole, PPD: paraphenylene diamine, ABCD: airborne contact dermatitis, CAD: chronic actinic dermatitis
| Case number | Age in years/sex | Occupation | Sites of dermatitis | Clinical diagnosis | Duration | Patch test results (grades) ISPTS kit 2015* |
Patch test results (grades) ISPTS kit 2018* |
|---|---|---|---|---|---|---|---|
| 1 | 70/malea | Agriculture | Face, neck and hands | Acrofacial dermatitis | 8 years | Parthenium (3+), neomycin (1+), fragrance mix (1+), formaldehyde (1+) andbenzocaine (1+) | Parthenium (3+), neomycin (1+), fragrance mix (1+), formaldehyde (1+)and chlorocresol (1+) |
| 2 | 52/male | Agriculture | Face, V area-neck and dorsal hands/feet | CAD | 10 years | Parthenium (3+u) and thiuram mix (3+) | Parthenium (3+), formaldehyde (2+) and 2MBT (2+) |
| 3 | 61/female | Homemaker/agriculture | Scalp dermatitis | CD from hair color | 6 months | All negative | All negative |
| 4 | 54/male | Agriculture/mason (part time) | Face, hands and feet | Acrofacial dermatitis | 4 years | Parthenium (2+) | Parthenium (2+) |
| 5 | 40/male | Agriculture | Face, neck and limb flexures | ABCD | 1 year | All negative | All negative |
| 6 | 61/maleb | Agriculture | Scalp, face, V area-neck and dorsal hands/feet | CAD | 10 years | Parthenium (3+), pot. dichromate (3+),benzocaine (1+), thiuram mix (2+), nitrofurazone (2+) and black rubber mix (3+) | Parthenium (3+), pot. dichromate (3+) and chlorocresol (3+) |
| 7 | 62/male | Self-employed gardening (spare time activity) | Face | Facial dermatitis | 3 months | Epoxy resin (3+), | Epoxy resin (3+), |
| 8 | 63/male | Agriculture | Face, neck, upper back and forearms (extensors) | CAD | 25 years | parthenium (3+), nitrofurazone (2+) andfragrance mix (1+) | parthenium (3+), nitrofurazone (2+), fragrance mix (1+),paraben (2+) andcolophony (1+) |
| 9 | 60/female | Home maker/agriculture | Face, chest, dorsal hands, forearms and legs (extensors) | CAD | 6 months | PPD (1+) andfragrance mix (1+) | PPD (1+) andfragrance mix (1+) |
| 10 | 47/femalec | Home maker/agriculture | Face, neck and trunk upper limbs | ABCD with photo aggravation | 15 years | Parthenium (3+), wool alcohol (2+), thiuram mix (1+) and2 MBT (2+) | Parthenium (3+), wool alcohol (1+) andthiuram mix (2+) |
Positive results shown in bold indicate allergens common to both kits. “3+ u” indicates intense positive reaction with ulceration. *All positive results were of definite clinical relevance, aPatch test results in 2010: parthenium (2+), bPatch test results in 2014: parthenium (1+), cPatch test results in 2010: parthenium (3+). Baseline characteristics: Males: Females 8:2; Age: 40-70 (mean 57.0) years; Duration of dermatitis: 3 months-25 (mean 7.43) years; Clinical patterns of dermatitis: ABCD in two patients, CAD in five patients, facial or acrofacial dermatitis in two patients and scalp dermatitis in one patient. ISPTS: Indian standard patch test series, MBT: mercaptobenzothiazole, PPD: paraphenylene diamine, ABCD: airborne contact dermatitis, CAD: chronic actinic dermatitis
| Case number | Age in years/sex | Occupation | Sites of dermatitis | Clinical diagnosis | Duration | Patch test results (grades) ISPTS kit 2014* |
Patch test results (grades) ISPTS kit 2018* |
|---|---|---|---|---|---|---|---|
| 1 | 73/male | Agriculture | Dorsal hands and feet, forearms (extensors) | Acral dermatitis | 1 year | Chlorocresol (1+) | Chlorocresol (1+) |
| 2 | 65/male | Agriculture | Face and V-area-neck | CAD | 10 years | Parthenium (3+u), 2MBT (1+), colophony (1+) andneomycin (1+) | Parthenium (3+) |
| 3 | 29/female | Homemaker/teacher | Face and dorsal hands | Acrofacial dermatitis | 1 year | All negative | All negative |
| 4 | 45/male | Agriculture | Face and thighs | Acrofacial dermatitis | 6 months | PPD (2+) | PPD (2+) andcobalt sulfate (1+) |
| 5 | 54/malea | Agriculture | Face, neck and upper limb extensors | CAD | 10 year | Angry back phenomenon | Angry back phenomenon |
| 6 | 55/maleb | Agriculture | Scalp, face, V-area-neck and dorsal hands/feet | CAD (cause hair color) | 1 year | All negative | All negative |
| 7 | 62/male | Self- employed | Face, neck and upper back | CAD | 1 year | PPD (1+) and fragrance mix (1+) | PPD (1+) |
| 8 | 43/female | Home maker/agriculture | Face, V area-neck, upper back and forearms (extensors) | CAD (cause hair color) | 5 years | PPD (2+) | PPD (2+) |
| 9 | 37/male | Mason/agriculture | Face and neck | ABCD | 3 years | All negative | All negative |
| 10 | 47/male | Agriculture | Face, neck and dorsal hands | CAD | 6 years | Parthenium (3+), Myroxylon pereirae (2+), pot. dichromate (1+), paraben mix (1+), fragrance mix (2+), thiuram mix (1+), nickel sulfate (1+), cobalt sulfate (1+) andwool alcohol (1+) | Parthenium (3+), Myroxylon pereirae (1+), pot. dichromate (1+), paraben mix (1+), fragrance mix (2+), thiuram mix (3+), epoxy resin (1+), formaldehyde (1+), nitrofurazone (1+) and PPD (1+) |
Positive results shown in bold indicate allergens common to both kits. “3+ u” indicates intense positive reaction with ulceration. *All positive results were of definite clinical relevance, aDeveloped angry back phenomenon, bShowed positive patch test results from his own hair color. Baseline characteristics: Males: Females 8:2; Age: 29-73 (mean 51.0) years; Duration of dermatitis: 6 months-10 (mean 3.85) years; Clinical patterns of dermatitis: ABCD in one patient, CAD in six patients, acrofacial dermatitis in two patients and acral dermatitis in one patient. ISPTS: Indian standard patch test series, MBT: mercaptobenzothiazole, PPD: paraphenylene diamine
| Patch test allergen | Concentration used of patch test allergen | Number of patients with positive results (Group-1) | Number of patients with positive results (Group-2) | Number of patients with positive results (Group-3) | |||
|---|---|---|---|---|---|---|---|
| Year of expiry | |||||||
| 2016 | 2018 | 2015 | 2018 | 2014 | 2018 | ||
| Vaseline | 100% petrolatum | 0 | 0 | 0 | 0 | 0 | 0 |
| Wool alcohols | 30.0% petrolatum | 0 | 1 | 1 | 1 | 1 | 0 |
| Myroxylon pereirae (Balsam of Peru) | 25.0% petrolatum | 1 | 3 | 0 | 0 | 1 | 1 |
| Formaldehyde | 1.0% petrolatum | 2 | 1 | 1 | 2 | 0 | 1 |
| MBT | 2.0% petrolatum | 1 | 1 | 1 | 1 | 1 | 0 |
| Potassium dichromate | 0.5% petrolatum | 0 | 0 | 1 | 1 | 1 | 1 |
| Nickel sulphate | 5.0% petrolatum | 0 | 0 | 0 | 0 | 1 | 0 |
| Cobalt sulphate | 1.0% petrolatum | 1 | 0 | 0 | 0 | 1 | 1 |
| Colophony | 20.0% petrolatum | 4 | 4 | 0 | 1 | 1 | 0 |
| Epoxy resin | 1.0% petrolatum | 0 | 1 | 1 | 1 | 0 | 1 |
| Paraben | 15.0% petrolatum | 1 | 1 | 0 | 1 | 1 | 1 |
| PPD | 1.0% petrolatum | 2 | 2 | 1 | 1 | 3 | 4 |
| Parthenium | 1.0% aqueous | 9 | 9 | 6 | 6 | 2 | 2 |
| Neomycin sulphate | 20.0% petrolatum | 1 | 0 | 1 | 1 | 1 | 0 |
| Benzocaine | 5.0% petrolatum | 2 | 1 | 2 | 0 | 0 | 0 |
| Chlorocresol | 1.0% petrolatum | 2 | 1 | 0 | 2 | 1 | 1 |
| Fragrance mix | 8.0% petrolatum | 3 | 3 | 3 | 3 | 2 | 1 |
| Thiuram mix | 1.0% petrolatum | 3 | 3 | 3 | 1 | 1 | 1 |
| Nitrofurazone | 1.0% petrolatum | 3 | 2 | 2 | 1 | 0 | 1 |
| Black rubber mix | 0.6% petrolatum | 2 | 2 | 1 | 0 | 0 | 0 |
| Total positive patch test results* | 37 | 35 | 24 | 23 | 18 | 16 | |

- Group 1, Case 8, positive patch test reactions from parthenium, colophony and thiuram mix were observed from haptens in both kits. Interestingly, parthenium from kit expired in 2016 elicited extreme positive reaction (3+ with ulceration) while reaction from thiuram mix was stronger (2+) compared to 1+ from the new kit. Nitrofurazone from expired kit was another clinically relevant allergen (sensitization was from medicated strips frequently used by him). However, only mercaptobenzothiazole, wool alcohol and fragrance mix from new kit elicited positive reactions
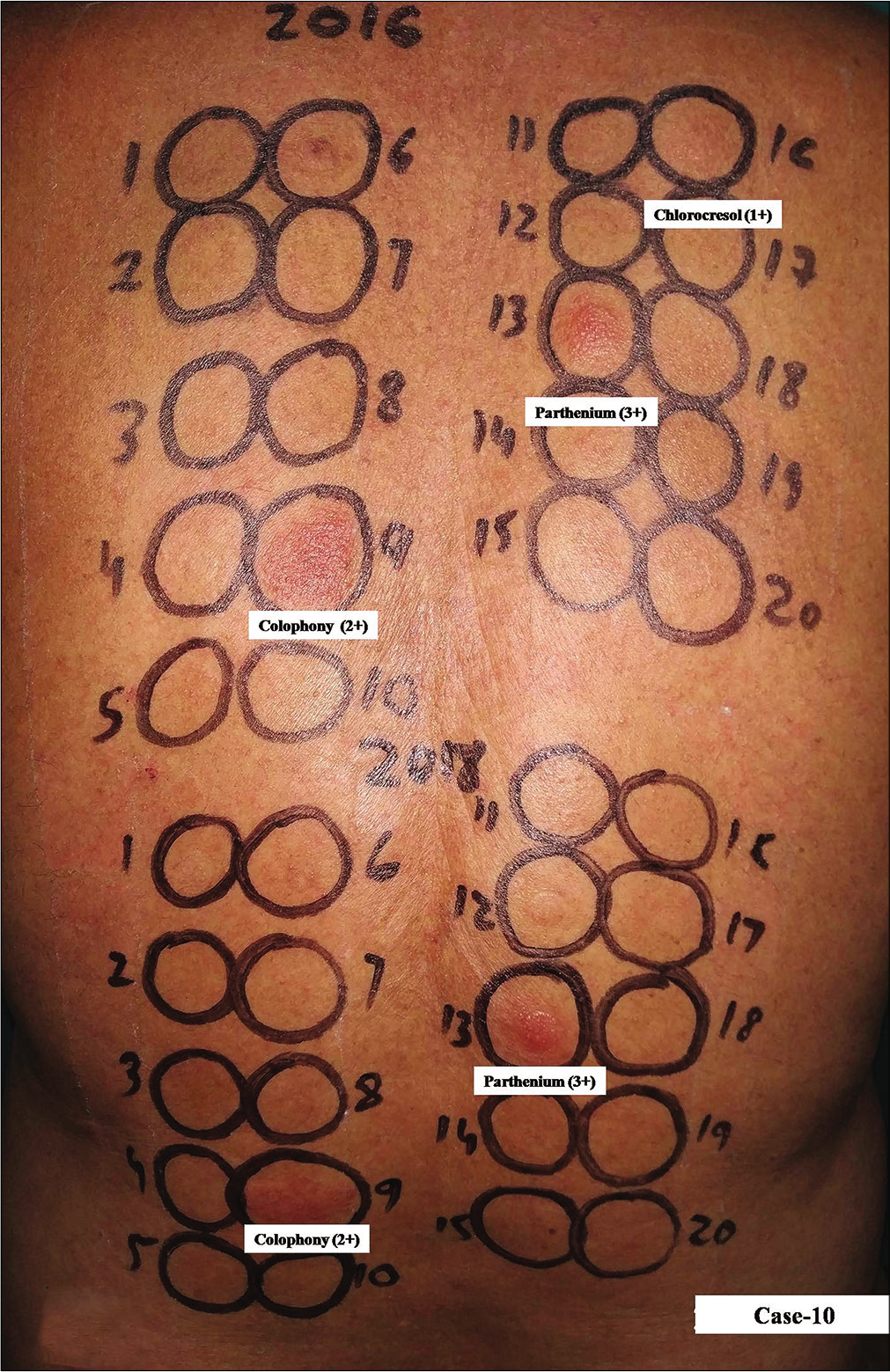
- Group 1, Case 10, positive patch test reactions of equal intensity were observed due to parthenium and colophony from both kits. Parthenium from kit expired in 2016 elicited stronger positive reaction (3+) compared to that from the new kit while colophony elicited positive reaction (2+) of equal intensity. Cholorocresol (a fungicide/antiseptic in topical medications) from the expired kit was another relevant allergen eliciting positive reaction
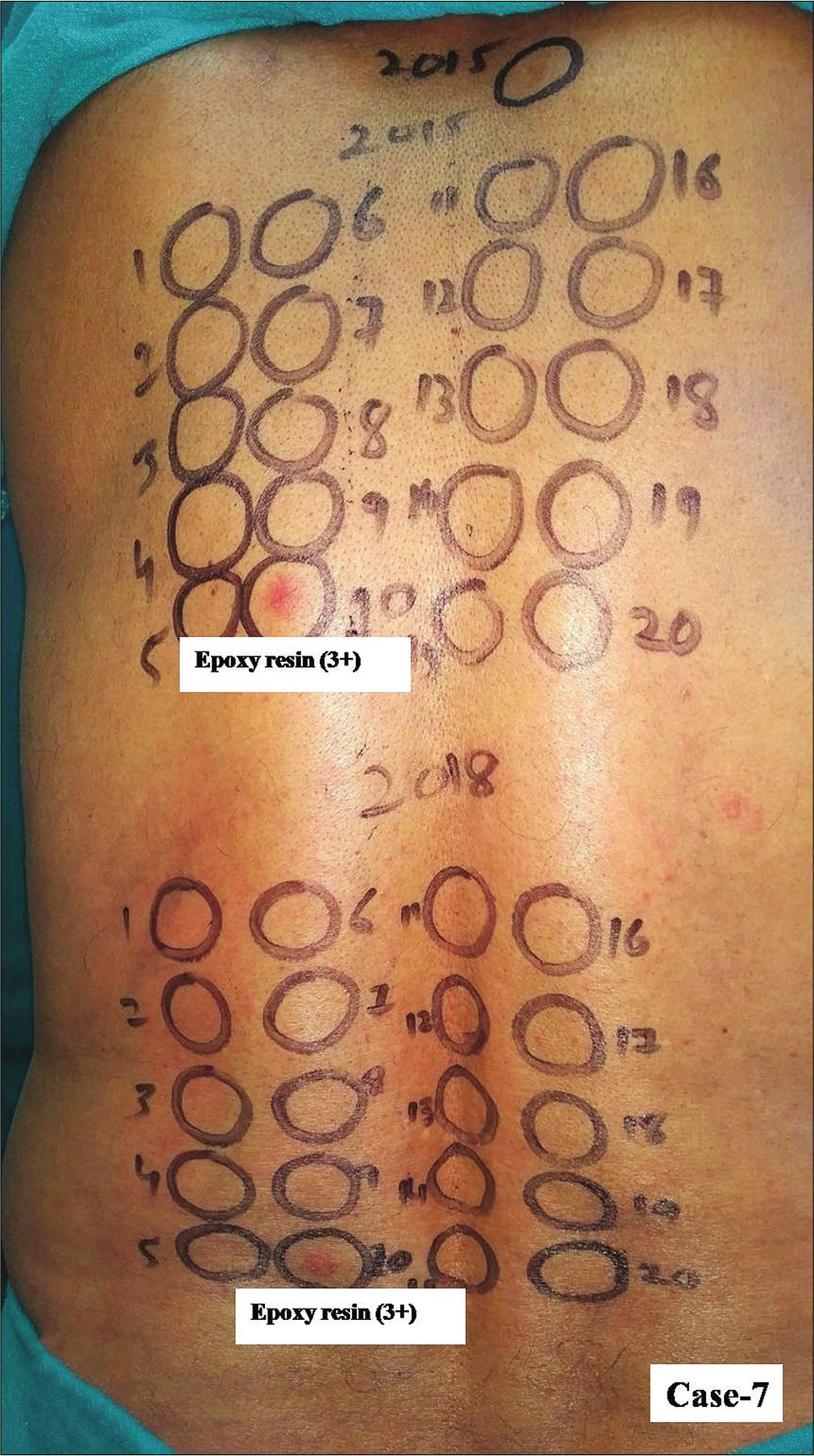
- Group 2, Case 7, positive patch test reactions of equal intensity were observed from epoxy resin from both kits
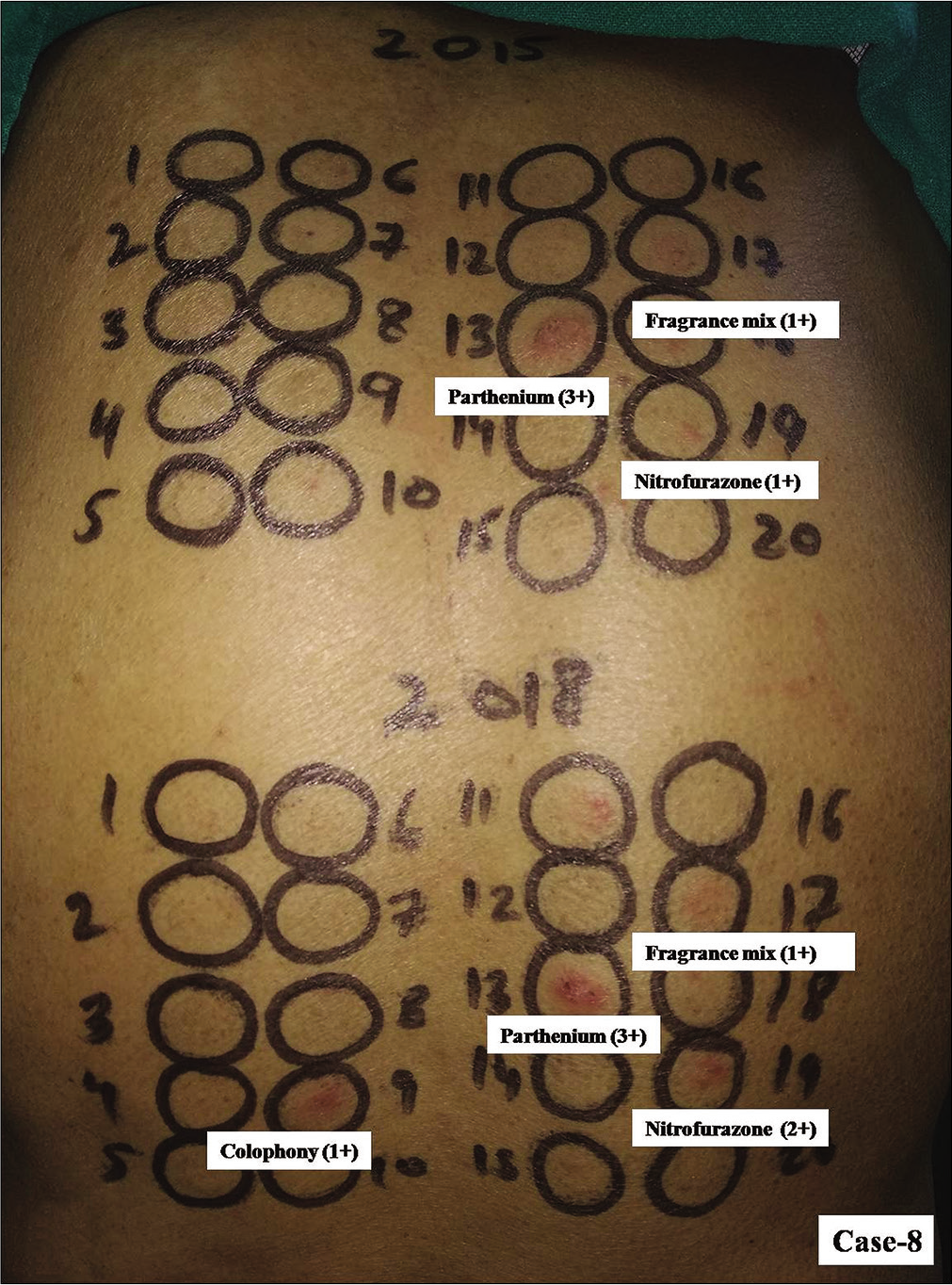
- Group 2, Case 8, positive patch test reactions of equal intensity were observed due to parthenium and fragrance mix from both kits. Colophony elicited positive reaction (1+) from kit expired in 2015 was another relevant allergen eliciting positive reaction in him
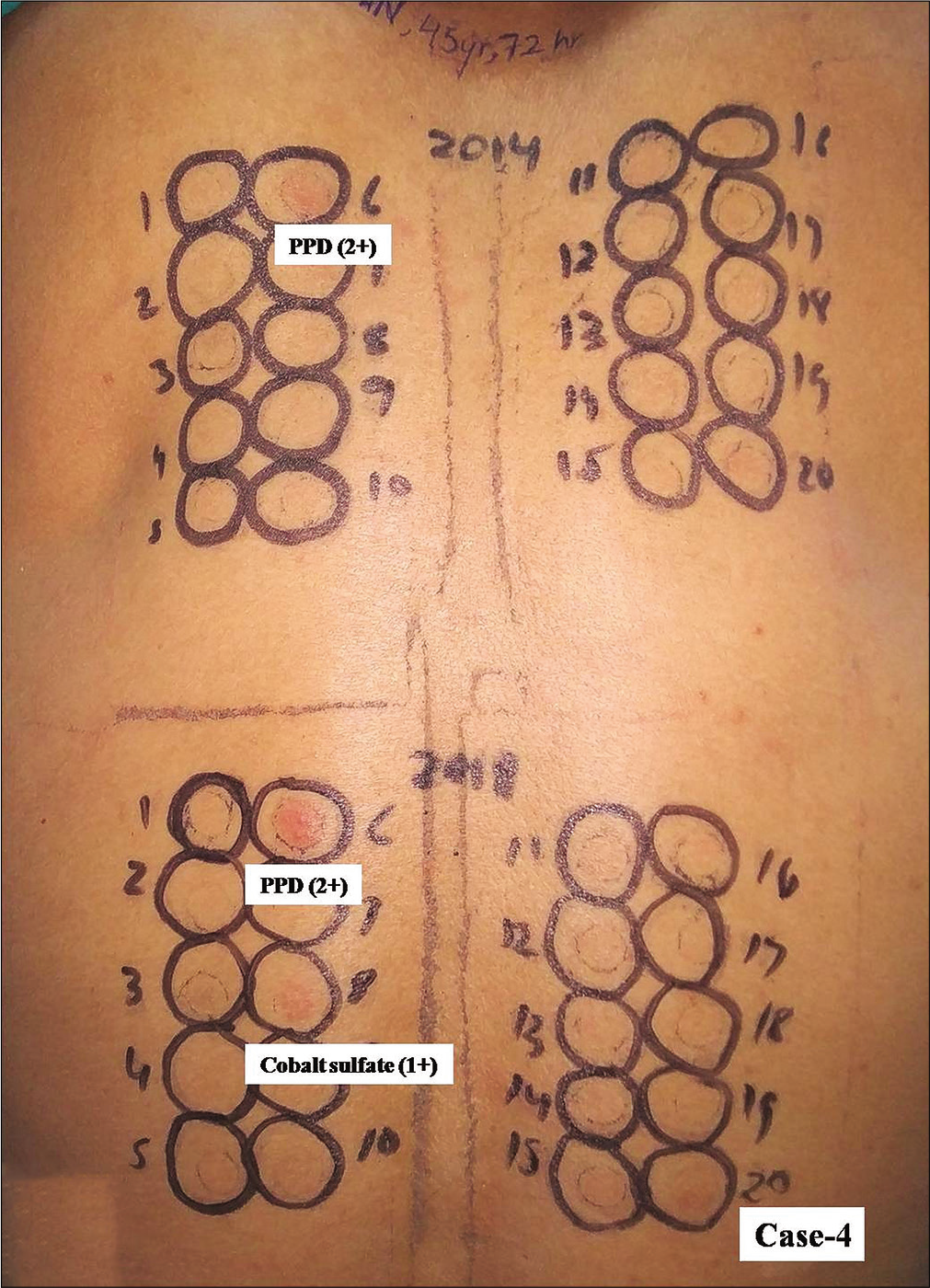
- Group 3, Case 4, positive patch test reactions from paraphenylene diamine occured from haptens in both kits. However, only cobalt sulfate from new kit elicited positive reaction
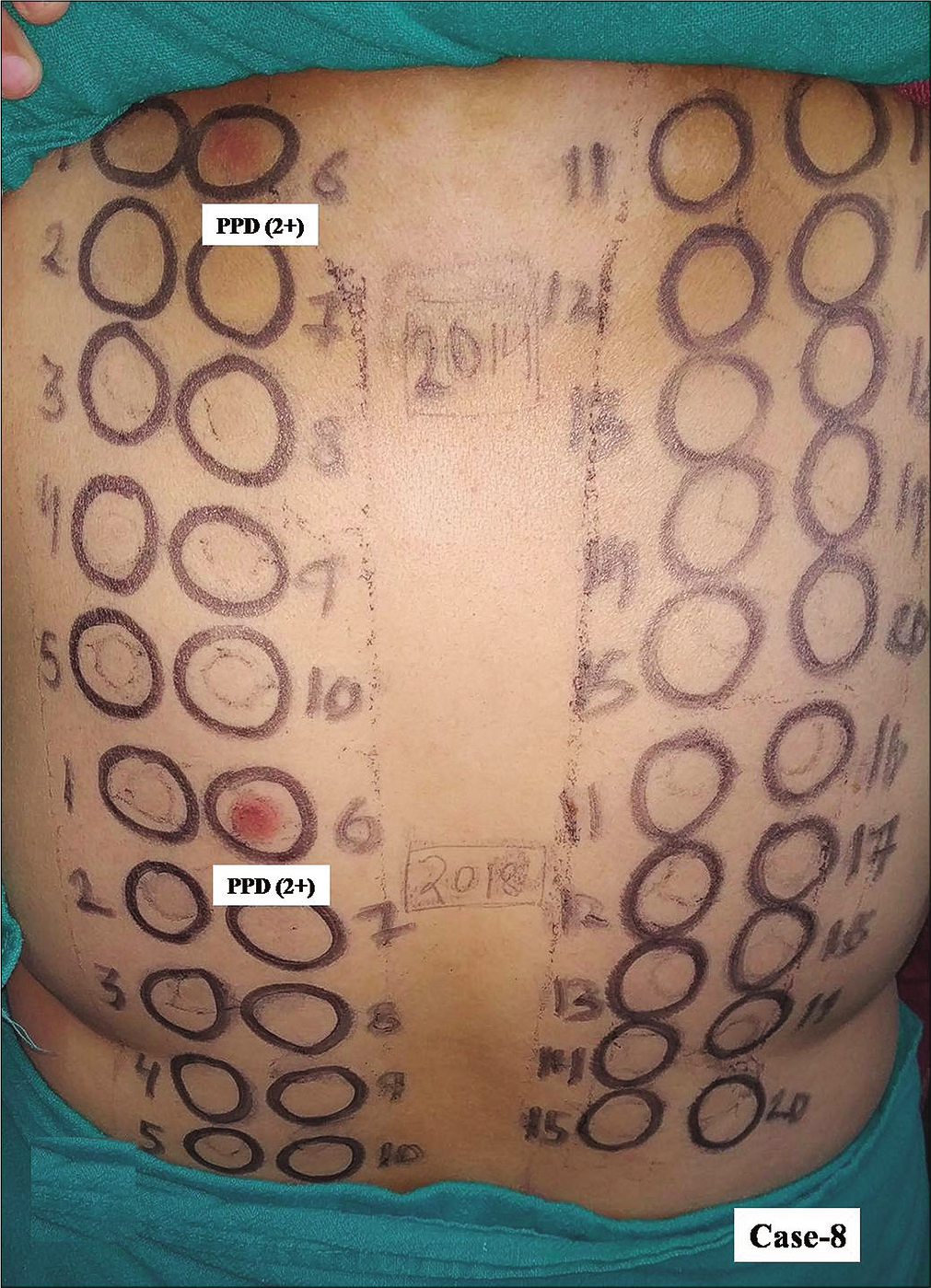
- Group 3, Case 8, positive patch test reactions of equal intensity (2+) were observed due to paraphenylene diamine in a patient with contact dermatitis from hair colorant
Discussion
Over the years, Contact Dermatitis and Occupational Dermatoses Forum of India-approved Indian standard patch test kit has become established across the country for patch testing.3-7 The reliability of patch testing with expired patch test kits has been not evaluated before. Parthenium, paraphenylene diamine, thiuram mix and black rubber mix are potent and ubiquitous contact sensitizers which was seen in the subjects of this study as well. Myroxylon pereirae, fragrances, colophony, preservatives, other prominent contact allergens in our patients, are common additives in topical medications leading to contact sensitization from their frequent use by dermatology patients. All these have been frequently documented allergens in the region.6,7 The positive reactions from patch test kits with expiry in 2016, 2015 and 2014 were from almost identical allergens and were comparable in intensity with those from the patch test kit with expiry in 2018. Fragrance mix and/or parthenium from both the kits also elicited positive reactions in one patient (case-3 in group 1) who had positive reactions from them in the past as well. Angry back or phenomenon of skin hyperreactivity in a group-3 patient from both the patch test kits reflected a potential to cause exaggerated true sensitivity by the expired kit as well.
It has been demonstrated that fragrances, acrylates or other volatile allergens are unstable and their concentration likely to vary from storage at room temperature affecting their allergenic potential.8,9 However, acrylates/methylacrylates in syringes retain their allergenic potential above 80% compared with IQTM chambers when stored in a plastic bag in a refrigerator.10 Interestingly, parthenium from all the kits showed equally intense positive reactions, contrary to the belief that its aqueous extract, like other volatile allergens deteriorates in a short time and tends to lose its potential to elicit positive patch test reactions.11 However, paraphenylene diamine, formaldehyde resin, paraben mix, benzocaine, epoxy resin, rubber allergens are nonvolatile allergens and remain relatively stable retaining their allergenic potential when stored well.12
Limitations
The small number of patients in each group remains the major limitation of the study. Sequential patch testing for true sensitivity reaction was not performed in patient with angry back phenomenon. We did not read results for late reactions. Whether or not these results can be extrapolated with patch test results from other similar patch test kits available across countries also needs confirmation.
Conclusion
Our observations reflect that most of the common patch test allergens including parthenium (aq) remain stable for long periods and a well stored Indian standard patch test kit can be used reliably even beyond the labeled expiry date, saving scarce economic resources. Since the safety of such kits may be a concern and relevance of patch test results from individual allergens in such a scenario perhaps remains questionable and requires careful interpretation, a few large studies are highly desirable before making any recommendations.
Acknowledgements
The authors sincerely thank Systopic India Ltd., New Delhi (India) for providing patch test kits with an expiry date. The authors also thank their patients/subjects who volunteered for the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- European Society of Contact Dermatitis guideline for diagnostic patch testing-recommendations on best practice. Contact Dermatitis. 2015;73:195-221.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of photopatch test allergens for Indian patients of photodermatitis: Preliminary results. Indian J Dermatol Venereol Leprol. 2011;77:148-55.
- [CrossRef] [PubMed] [Google Scholar]
- Patch testing experience with 1000 patients. Indian J Dermatol Venereol Leprol. 2007;73:313-8.
- [CrossRef] [PubMed] [Google Scholar]
- Contact sensitization in venous eczema: Preliminary results of patch testing with Indian standard series and topical medicaments. Indian J Dermatol Venereol Leprol. 2009;75:136-41.
- [CrossRef] [PubMed] [Google Scholar]
- A study on incidence of various allergens involved in allergic contact dermatitis by patch testing among 150 patients in a tertiary care hospital in South India. Int J Res Dermatol. 2018;4:205-10.
- [CrossRef] [Google Scholar]
- Occupational contact dermatitis among construction workers: Results of a pilot study. Indian J Dermatol Venereol Leprol. 2014;80:159-61.
- [CrossRef] [PubMed] [Google Scholar]
- Patch test results from a contact dermatitis clinic in North India. Indian J Dermatol Venereol Leprol. 2011;77:194-6.
- [CrossRef] [PubMed] [Google Scholar]
- Stability of selected volatile contact allergens in different patch test chambers under different storage conditions. Contact Dermatitis. 2012;66:172-9.
- [CrossRef] [PubMed] [Google Scholar]
- Stability of fragrance patch test preparations applied in test chambers. Br J Dermatol. 2012;167:822-7.
- [CrossRef] [PubMed] [Google Scholar]
- Variation in allergen content over time of acrylates/methacrylates in patch test preparations. Br J Dermatol. 2011;164:116-24.
- [CrossRef] [PubMed] [Google Scholar]
- Parthenium dermatitis: Is parthenolide an effective choice for patch testing? Contact Dermatitis. 2014;70:340-3.
- [CrossRef] [PubMed] [Google Scholar]
- Vapor pressure and predicted stability of American Contact Dermatitis Society Core Allergens. Dermatitis. 2016;27:193-201.
- [CrossRef] [PubMed] [Google Scholar]






