Translate this page into:
Pemphigus in North-Western Yemen: A therapeutic study of 75 cases
Correspondence Address:
Mishri Lal Khatri
Consultant Dermatologist, Saudi Hospital, Hajjah, P.O. Box: 2757, Sana'a
Republic of Yemen
| How to cite this article: Khatri ML. Pemphigus in North-Western Yemen: A therapeutic study of 75 cases. Indian J Dermatol Venereol Leprol 2016;82:359 |
Abstract
Background: The incidence of pemphigus, though not documented, seems to be quite high in Yemen. There is no universal consensus on the treatment of this disease. Aims: The aim was to evaluate the efficacy and side effects of different therapeutic regimens used in patients of pemphigus in North-Western Yemen. Patients and Methods: Seventy-five Yemeni patients (39 males and 36 females) were included. Diagnosis was based on clinical features, histopathology and the Tzanck test. Results of treatment with these different therapeutic regimens were compared: (1) dexamethasone-cyclophosphamide pulse (DCP), (2) dexamethasone pulse with oral azathioprine, (3) oral prednisolone with azathioprine, (4) oral prednisolone with oral cyclophosphamide, and (5) prednisolone monotherapy. Results: Pemphigus vulgaris (PV) was diagnosed in 46 patients, pemphigus foliaceus (PF) in 23, pemphigus vegetans (PVEG) in 5 and pemphigus herpetiformis (PH) in one. Among the 16 patients who received regular DCP therapy, 13 were in remission for 6 months to 11 years without medications (phase 4). Remission without pharmacotherapy could not be achieved with the other regimens and steroid-induced side-effects appeared to be more than with DCP. Limitations: Immunofluorescence was not available to confirm the diagnosis of pemphigus. Randomization was not done. Conclusion: The DCP regimen seemed to be superior to the other regimens used.Introduction
Pemphigus is a group of autoimmune blistering diseases mediated by antibodies directed against desmosomal adhesion proteins, varying in clinical features and incidence in various population groups.[1],[2] It can occur at any age, though most commonly in middle age, and both sexes are affected equally.[3]
The disease was almost always fatal before the advent of corticosteroids. Introduction of adjuvant therapy has reduced the side effects of long-term corticosteroid therapy and further improved the prognosis. In the absence of a universal consensus on the treatment of pemphigus, different treatment strategies are followed. A regimen is selected based on the age of the patient, the degree of involvement, rate of progression, type of pemphigus, other associated diseases if any, and treatment cost.
This is a prospective study of 75 cases of pemphigus treated and followed up over the last 17 years at the Department of Dermatology, Saudi Hospital at Hajjah, Republic of Yemen, run by the Government of Saudi Arabia.
Patients and Methods
All the patients were admitted for initial assessment and some re-admitted at recurrence of the disease. Details of history, physical findings, laboratory data, treatment and follow-up were recorded in a standardized protocol. The diagnosis was based on clinical features, histopathology and the Tzanck test. Immunofluorescence studies were not available at our centre or elsewhere in Yemen. All patients were screened for pulmonary tuberculosis, diabetes, hypertension, cardiac disease and other autoimmune diseases. Menstrual and obstetric history was recorded in all female patients.
Routine investigations including complete blood counts, blood sugar, liver and kidney function tests, serum electrolytes, routine urine and stool examinations with stool occult blood were performed initially. Complete blood counts, serum electrolytes, serum creatinine and blood sugar were checked daily for the seriously ill in-patients and at every follow-up (4 weeks) in out-patients. The other investigations were done every 3 months or when felt necessary during and after the treatment.
Our aim was to try dexamethasone-cyclophosphamide pulse (DCP) therapy in the as many patients as possible because of its known efficacy effects. However it could not be used in younger patients (children, unmarried patients and those who had not completed their family) because of its potential for gonadal suppression; dexamethasone pulse with oral azathioprine therapy was tried instead. Both these modalities could not be used in many patients who could not come to the hospital 3 days every month due to economic constraints, although all services and drugs are provided free of cost at our hospital. For such patients, oral prednisolone therapy with azathioprine was tried. A combination of prednisolone and cyclophosphamide was used in a few patients who did not respond to prednisolone with azathioprine and and also in those who did not tolerate azathioprine and DCP. Prednisolone monotherapy was used mainly for pregnant patients as it is comparatively safe, and in a few patients of pemphigus foliaceus with minimal lesions.
Disease control was defined as healing of all existing lesions with no new lesions appearing.
Protocols for treatment
Dexamethasone-cyclophosphamide pulse
Phase 1: Injection dexamethasone 100 mg in 500 ml of 5% dextrose, intravenously over 2 hours, repeated on 3 consecutive days, with injection cyclophosphamide 500 mg added to the drip on the second day. Such 3-day pulses were repeated every 4 weeks. In between the pulses, oral cyclophosphamide (50 mg daily) was advised. These cycles were continued till control of the disease. For early initial control of the disease, oral prednisolone was also given as follows: starting doses 40 to 60 mg/day according to disease severity, tapered by 5 mg every week till 20 mg/day was reached, then by 5 mg every two weeks till 10 mg was reached, followed 4 weeks later by tapering to 10 mg on alternate days for another 4 weeks, and then discontinued if no new lesions occurred).
Phase 2: Nine more DCPs were given as in phase 1 with daily 50 mg oral cyclophosphamide only in between the cycles
Phase 3: Oral cyclophosphamide 50 mg/day for another 9 months.
Phase 4: Follow-up without medications for 10 years.
Dexamethasone pulse with oral azathioprine
Phase 1: Injection dexamethasone 100 mg in 500 ml of 5% dextrose, intravenously over 2 hours, repeated on 3 consecutive days, every 4 weeks. In children below 12 years, half the above dose was given. In between pulses, oral azathioprine 100 mg/day in adults and 50 mg/day in children below 12 years and oral prednisolone (40 to 60 mg in adults and 20 to 30 mg in children) were given according to severity of the disease, with the prednisolone tapered (as above) to 10 mg every other day.
Phase 2: Another six dexamethasone pulses were given. In between the pulses, azathioprine at the same doses and prednisolone 10 mg every other day were continued.
Phase 3: Azathioprine 50 mg and prednisolone 10 mg every other day (both on the same day) were continued for 6 more months.
Phase 4: Follow-up with an attempt to withdraw the drugs at every 2 to 3 months. Both drugs were restarted at recurrence of lesions.
Oral prednisolone with azathioprine
Initially, prednisolone 2–3 mg/kg/day (80–120 mg in adults) and azathioprine 2–2.5 mg/kg/day (100–150 mg in adults) were advised. These were continued till control of the disease with the exception of the occasional appearance of one or two lesions. Medications were then tapered as follows: prednisolone tapered by 5 mg/week to 20 mg/day, followed by azathioprine tapering by 50 mg every 4 weeks to a dose of 50 mg/day. Further reductions of prednisolone (5 mg/4 weeks) to 10 mg every other day, then azathioprine down to 50 mg every other day were subsequently advised.
Oral prednisolone + oral cyclophosphamide
Prednisolone 2–3 mg/kg/day and oral cyclophosphamide 2 mg/kg/day were advised initially and the doses gradually tapered after disease control (as with prednisolone plus azathioprine, above).
Prednisolone monotherapy
Prednisolone 1–3 mg/kg/day initially with gradual tapering of the dose.
Written consent of the patient and wife/husband/father were taken after explaining the advantages and side effects of each therapy. For DCP therapy, a special information note in Arabic and English was provided before obtaining the consent.
Results
All 75 patients (39 (52%) males and 36 (48%) females) were from North-Western Yemen. The age at onset of the disease ranged between 10 and 85 years (median, 39.5 years). The incidence of pemphigus among new dermatology patients in our department was 0.1%.
On the basis of clinical features and histopathologic findings, the cases were classified as pemphigus vulgaris in 46 (61.3% of all cases; 22 male, 24 female), pemphigus foliaceus in 23 (30.6%; 14 male, 9 female), pemphigus vegetans in 5 (6.6%; 2 male, 3 female) and pemphigus herpetiformis in 1 (1.3%; male).
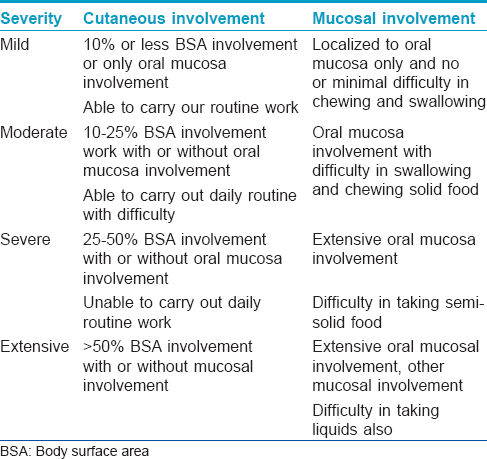
Patients were also classified as having mild (n = 4), moderate (13), severe (38) or extensive (20) disease, severity scoring being as in [Table - 1].
Associated diseases
At the initial presentation, 3 patients had diabetes mellitus and 3 were hypertensive. Three of these patients died during the course of this study, as described in the respective sections. One patient on DCP therapy, who had a history of treated pulmonary tuberculosis, developed reactivation of the disease. One patient had a hydatid cyst in the liver and there was no change in its status during treatment. All these associated conditions were managed with the supervision of the respective specialists. One patient had additional endogenous eczema, which improved.
Treatment and follow-up
Twenty patients (pemphigus vulgaris, 12, pemphigus foliaceus, 6, pemphigus vegetans, 1 and pemphigus herpetiformis, 1) had received no treatment before being seen at our centre. Five of them were started on DCP therapy. The others had previously had irregular treatment with prednisolone, and azathioprine in some cases.
Dexamethasone-cyclophosphamide pulse therapy
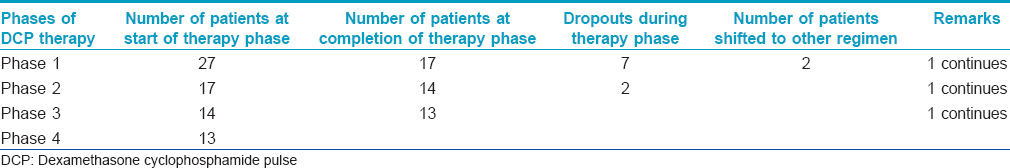
DCP therapy was initiated in 27 patients, (11 males and 16 females; pemphigus vulgaris 19, pemphigus foliaceus 7 and pemphigus vegetans 1). Involvement was extensive in 12, severe in 10 and moderate in 5 patients [Table - 2]. Of these, 9 patients dropped out in various phases of DCP; one was shifted to oral prednisolone with azathioprine as he was unable to attend the hospital for three days every month and another to oral prednisolone with oral cyclophosphamide as he did not tolerate the high intravenous dose of cyclophosphamide. The number of DCP cycles to control the disease (phase 1) ranged from 4 to 27 (median number 9) Out of the 16 patients who continued on the DCP regimen, 13 were on follow-up without treatment (phase 4) for 6 months to 11 years without recurrence of the disease at the time of writing this report. Two patients had recurrences 4 and 13 months into phase 4. DCP was repeated in these patients and they were subsequently in remission for 1 and 3 years respectively (Phase 4 after 2nd course of DCP). The treatment status of patients on DCPs is summarized in [Table - 2].
Intravenous immunoglobulin (IVIG) was used as adjuvant therapy in two patients with severe pemphigus vulgaris who initially responded poorly to DCP. One patient was given two cycles of 100 g IVIG each, 2 weeks apart, after the second DCP cycle. There was a dramatic improvement in his condition and disease clinically cleared with another 2 cycles of DCP. The other patient was given two cycles of 40 g IVIG each, 2 weeks apart after the sixth cycle of DCP and this patient also improved rapidly. A lower dose of IVIG had to be administered because sufficient quantities were unavailable. DCPs were then continued and disease activity was controlled with 13 cycles (Phase 1) in this patient. Both these patients were on phase 2 DCP therapy at the time of this report.
The number of DCP cycles to control the disease for clinical clearance (Phase 1) ranged from 4 to 27 (median, 9).
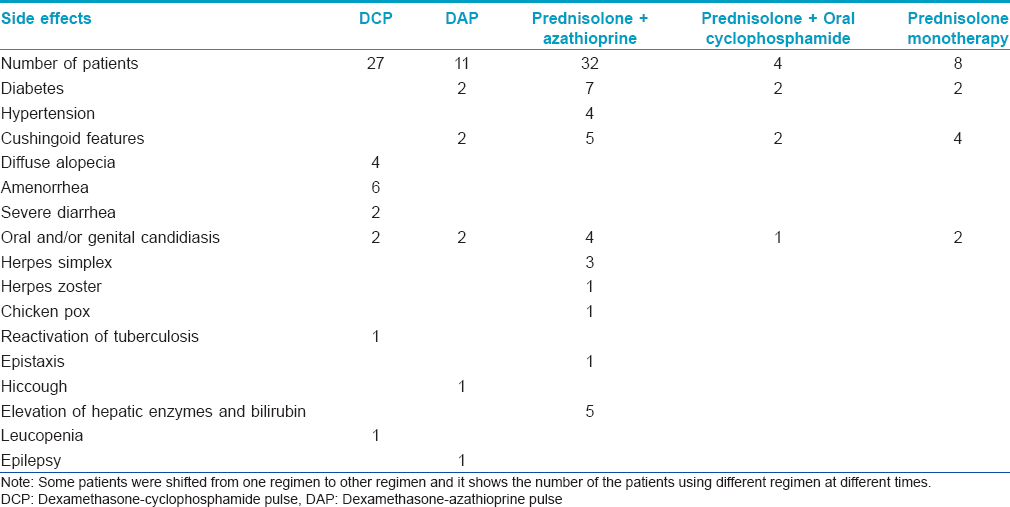
Side effects due to DCP are listed in [Table - 3]. Two patients had diabetes and 4 had Cushingoid features before starting DCP due to previous prolonged use of steroids.
Dexamethasone pulse with oral azathioprine
Initially, we had planned the protocol of this combination to be analogous to DCP, including withdrawing prednisolone completely in the first phase. However, recurrences on discontinuation of the small doses of prednisolone necessitated the regimen described above instead. This therapy was initiated in 11 younger patients (8 males and 3 females; pemphigus vulgaris 6, pemphigus foliaceus 4 and pemphigus vegetans 1). The disease was controlled / cleared in 4–8 cycles in 8 patients (Phase 1). Two patients dropped out in various phases and 3 were shifted to an oral prednisolone plus azathioprine regimen as they were unable to spare three days for the pulse. Four patients were in phase 4 follow-up for 1–2 years. Attempts to withdraw medications resulted in recurrences with a few lesions; maintenance doses of azathioprine 50 mg and prednisolone 10 mg on alternate days kept the disease under control.
Two patients in this group died. One patient aged 20 years having erythrodermic pemphigus foliaceus apparently improved after three pulse cycles but suddenly died 2 days after the third pulse while on oral prednisolone 40 mg and azathioprine 100 mg/day. The cause of death was determined to be cardiorespiratory arrest probably due to aspiration. He did not have any known cardiac disease before this. The other patient aged 25 years had extensive pemphigus vulgaris when he first presented to us. He had previously been on irregular treatment with oral prednisolone and azathioprine. He too improved clinically after 3 pulse cycles but discontinued all treatment and follow-up after that. When he returned after three months, he had extensive skin involvement with secondary infection and died after 8 days due to sepsis.
Pus culture yielded p seudomonas species, though a blood culture was negative in this case.
Oral prednisolone with azathioprine
This therapy was initiated in 32 patients (16 male and 16 female; pemphigus vulgaris 22, pemphigus foliaceus 8 and pemphigus vegetans 2). At the time of initial assessment, diabetes and hypertension were detected in 2 patients and hypertension alone in one more patient. One patient was epileptic. Two patients did not come for follow-up after initial assessment. Four patients did not show significant improvements at 6 months and more, so 3 of them were shifted to the DCP regimen and one to the prednisolone and oral cyclophosphamide regimen. The remaining 26 patients responded well with disease control in 3 months to 1 year, and were then kept on maintenance treatment which varied from azathioprine 50 mg plus prednisolone 10 mg on alternate days, to daily dosing. Two patients with pemphigus vulgaris and one with pemphigus vegetans had remissions for 2–3 years without treatment. One of them (pemphigus vulgaris) came with a recurrence after 3 years, was treated again for 2 years and had a subsequent remission for 2 years without therapy. Ten patients continued regular follow-up and were under remission with the maintenance dose. The remaining 16 patients were not regular and came with recurrences over a 5-year period. One woman with pemphigus vulgaris was controlled after 1 year of treatment and then lost to follow-up and she returned after 5 years with multiple vegetative lesions and moderate oral involvement.
Three patients in this group died. One, a 70-year-old man with pemphigus vulgaris and a known hypertensive, who had had the disease controlled and was on maintenance treatment, died due to a stroke 1 year after the initiation of treatment. Another 67-year-old patient of pemphigus foliaceus and a known diabetic was started on treatment with full-dose azathioprine and low-dose prednisolone (40 mg/day) after controlling his blood sugars. The patient died due to diabetic ketoacidosis after 1 month. The third patient with pemphigus vulgaris and hypertension died at home probably due to ischemic heart disease.
Prednisolone with oral cyclophosphamide
Three patients who did not respond well despite prolonged therapy (12 to 18 months) with prednisolone and azathioprine and one patient who did not tolerate DCP were given this combination therapy. In 2 of them, disease activity was controlled in 3–6 months. One patient who did not show a good response had to be shifted to DCP therapy, which he had initially declined. His disease was then controlled and he was in phase 4 of DCP. The fourth patient in this group was lost to follow-up after 2 months.
Prednisolone monotherapy
This therapy was initiated in 3 patients with mild pemphigus foliaceus and one with pemphigus herpetiformis. Three of these had their disease partially controlled by 3–6 months but were then lost to follow-up. One patient who did not respond well was shifted to DCP therapy.
Three other patients of pemphigus vulgaris received prednisolone monotherapy in pregnancy. Two of them presented at 3 and 6 months of gestation respectively, while the third conceived while on prednisolone alone, azathioprine having been stopped after her marriage. All 3 had partial disease control with moderate doses of prednisolone. One of the babies delivered died soon after birth. The other 2 patients continued on prednisolone through lactation. Another patient with pemphigus vegetans received moderate doses of prednisolone during 3 pregnancies. One of the babies died a month after birth. All these patients were shifted back to their original therapy with prednisolone and azathioprine subsequently. One of them (pemphigus vulgaris) developed hyperbilirubinemia later and was shifted to DCP therapy.
Side effects of all the regimens are listed in [Table - 3].
Discussion
Pasricha et al. reported long-term remission/cure in 84% of their cases treated with DCP therapy.[4] Different studies on DCP therapy from India have revealed disease remission in 40–100% of the cases after 8–48 months' therapy. Other advantages noticed in numerous previous studies were rapid healing, reduced morbidity and lower incidence of common side effects of corticosteroids.[4],[5],[6],[7],[8],[9] Results in our DCP-treated cases were similar to those in previous studies.[4],[5],[6],[7],[8],[9] We did not find any previous report of treating pemphigus with DCP therapy in the Middle East. One group treated most of their patients of pemphigus in Kuwait with prednisolone monotherapy using adjuvants like azathioprine, oral cyclophosphamide and dapsone in moderately severe cases who did not respond.[10]
Intravenous immunoglobulin has been used successfully as an adjuvant therapy in pemphigus, either alone or in combination with cyclophosphamide. The mechanism of action of IVIG in most autoimmune diseases remains uncertain. In pemphigus, it seems to work by rapidly, dramatically and selectively lowering serum levels of pemphigus autoantibodies. Previous studies revealed that 1 week after a single cycle of IVIG, the level of pemphigus autoantibodies decreased by 70%.[11],[12] We were unable to find any previous report of using IVIG with DCP therapy. In two of our serious cases of pemphigus vulgaris who were not responding well to DCP therapy, we used IVIG as an adjuvant. Their response was dramatic and we feel that if IVIG is added to DCP in difficult cases it might reduce the duration of the therapy.
Gonadal suppression, especially premature menopause, is a major concern with DCP therapy in younger patients. A randomized trial of triptorelin (a gonadotropin-releasing hormone analogue) in premenopausal breast cancer patients on chemotherapy concluded that triptorelin induces temporary ovarian suppression during chemotherapy and reduces the occurrence of chemotherapy-induced early menopause.[13] It is possible that triptorelin may reduce the incidence of premature menopause due to cyclophosphamide in premenopausal pemphigus patients on DCP as well; a trial to investigate this aspect may be worthwhile.
The British Association of Dermatologists' recommends azathioprine as a well-established adjuvant drug in pemphigus. It has been used in doses of 1–3 mg/kg/day, though it should ideally be titrated according to the activity of thiopurine methyltransferase (TPMT).[14] Superior results of combination therapy with azathioprine and oral prednisolone as compared to prednisolone monotherapy have been reported in the past.[15]
Dexamethasone pulses with azathioprine as a variation of pulse therapy has been reported in 4 patients of pemphigus, with azathioprine 50 mg daily replacing cyclophosphamide during the first three phases without azathioprine boluses during the pulses.[16] Three of these patients achieved treatment-free remissions in the last 24 months of follow-up, though the fourth patient did not respond even after 16 cycles. Our experience with this modality was however somewhat different in that although 8 patients were controlled with 4–8 cycles, we could not withdraw azathioprine completely despite using higher doses (100 mg/day). All our patients on prednisolone and azathioprine who continued regular follow-up too needed continued maintenance treatment, though 3 were able to stop medications for 2-3 years in between. Side effects in this group seemed to be more frequent than with the DCP regimen, and 3 patients died due to possibly unrelated causes. Although we could not achieve complete remission without drugs in the dexamethasone pulse plus azathioprine regimen, we feel that it had some advantages over combining oral prednisolone with azathioprine. Disease control seems to be earlier with the former and the dose of prednisolone could be minimized early, and this may have resulted in the lower frequency of side effects noted.
We treated all 4 pregnant patients in this study with moderate doses of prednisolone alone, with which they were partially controlled. Neonatal deaths occurred in 2 of these cases, recurring thrice in one. Others have treated most of their pregnant pemphigus patients with prednisolone monotherapy with good results.[17],[18] Eight pregnant pemphigus vulgaris patients treated successfully with IVIG without any significant side effects have also been reported.[19]
Limitations
Immunofluorescence was not available to confirm the diagnosis of pemphigus. Randomization was not done.
To conclude, there seemed to be early control, longer remissions and less frequent side effects with DCP therapy as compared to the other regimens used.
We feel that if gonadal suppression due to cyclophosphamide can be reduced by the use of triptorelin and DCP is supplemented with IVIG, this modality can be used in younger patients and the duration of the therapy reduced, though these aspects need to be studied.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol 1999;40:167-70.
[Google Scholar]
|
| 2. |
Jamora MJ, Jiao D, Bystryn JC. Antibodies to desmoglein 1 and 3, and the clinical phenotype of pemphigus vulgaris. J Am Acad Dermatol 2003;48:976-7.
[Google Scholar]
|
| 3. |
Joly P, Litrowski N. Pemphigus group (Vulgaris, vegetans, foliaceus, herpetiformis, brasiliensis). Clin Dermatol 2011;29:432-6.
[Google Scholar]
|
| 4. |
Pasricha JS, Khaitan BK, Raman RS, Chandra M. Dexamethasone-cyclophosphamide pulse therapy for pemphigus. Int J Dermatol 1995;34:875-82.
[Google Scholar]
|
| 5. |
Pasricha JS, Poonam. Current regimen of pulse therapy for pemphigus: Minor modifications, improved results. Indian J Dermatol Venereol Leprol 2008;74:217-21.
[Google Scholar]
|
| 6. |
Roy R, Kalla G. Dexamethasone: Cyclophosphamide pulse (DCP) therapy in pemphigus. Indian J Dermatol Venereol Leprol 1997;63:354-6.
[Google Scholar]
|
| 7. |
Kandan S, Thappa DM. Outcome of dexamethasone- cyclophosphamide pulse therapy in pemphigus: A case series. Indian J Dermatol Venereol Leprol 2009;75:373-8.
[Google Scholar]
|
| 8. |
Kanwar AJ, Kaur S, Thami GP. Long-term efficacy of dexamethasone-cyclophosphamide pulse therapy in pemphigus. Dermatology 2002;204:228-31.
[Google Scholar]
|
| 9. |
Mahajan VK, Sharma NL, Sharma RC, Garg G. Twelve-year clinico-therapeutic experience in pemphigus: A retrospective study of 54 cases. Int J Dermatol 2005;44:821-7.
[Google Scholar]
|
| 10. |
Alsaleh QA, Nanda A, Al-Baghli NM, Dvorak R. Pemphigus in Kuwait. Int J Dermatol 1999;38:351-6.
[Google Scholar]
|
| 11. |
Bystryn JC, Rudolph JL. IVI g treatment of pemphigus: How it works and how to use it. J Invest Dermatol 2005;125:1093-8.
[Google Scholar]
|
| 12. |
Czernik A, Beutner EH, Bystryn JC. Intravenous immunoglobulin selectively decreases circulating autoantibodies in pemphigus. J Am Acad Dermatol 2008;58:796-801.
[Google Scholar]
|
| 13. |
Del Mastro L, Boni L, Michelotti A, Gamucci T, Olmeo N, Gori S, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: A randomized trial. JAMA 2011;306:269-76.
[Google Scholar]
|
| 14. |
Harman KE, Albert S, Black MM, British Association of Dermatologists. Guidelines for the management of pemphigus vulgaris. Br J Dermatol 2003;149:926-37.
[Google Scholar]
|
| 15. |
Aberer W, Wolff-Schreiner EC, Stingl G, Wolff K. Azathioprine in the treatment of pemphigus vulgaris. A long-term follow-up. J Am Acad Dermatol 1987;16:527-33.
[Google Scholar]
|
| 16. |
Rao PN, Lakshmi TS. Pulse therapy and its modifications in pemphigus: A six year study. Indian J Dermatol Venereol Leprol 2003;69:329-33.
[Google Scholar]
|
| 17. |
Kardos M, Levine D, Gürcan HM, Ahmed RA. Pemphigus vulgaris in pregnancy: Analysis of current data on the management and outcomes. Obstet Gynecol Surv 2009;64:739-49.
[Google Scholar]
|
| 18. |
Daneshpazhoo M, Chams-Davach C, Valikhani M, Aghabagheri A, Mortazavizadeh SM, Barzegari M, et al. Pemphigus and pregnancy: A 23 years experience. Indian J Dermatol Venereol Leprol 2011;77:534.
[Google Scholar]
|
| 19. |
Ahmed AR, Gürcan HM. Use of intravenous immunoglobulin therapy during pregnancy in patients with pemphigus vulgaris. J Eur Acad Dermatol Venereol 2011;25:1073-9.
[Google Scholar]
|
Fulltext Views
2,065
PDF downloads
1,964





