Translate this page into:
Pirfenidone induced phototoxic reaction in an elderly man
2 Department of Dermatology, Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India
Correspondence Address:
Rohini Pradip Gaikwad
Flat Number 5, Hindustan Arcade, Tingrenagar Road, Vishrantvadi, Pune - 411 015, Maharashtra
India
| How to cite this article: Gaikwad RP, Mukherjee SS. Pirfenidone induced phototoxic reaction in an elderly man. Indian J Dermatol Venereol Leprol 2016;82:101-103 |
Sir,
Pirfenidone is an antifibrotic and anti-inflammatory agent used in the treatment of idiopathic pulmonary fibrosis. It reduces fibroblast proliferation, inhibits transforming growth factor-β stimulated collagen production and reduces the production of fibrogenic mediators. Although photosensitivity and rash are reported side effects in clinical trials, we were able to find only a few previously published reports of this adverse effect.[1]
A 70-year-old man presented with burning sensation and a rash for 20 days. He had intense erythema with desquamation involving the face, scalp [Figure - 1], V area of the chest, shoulder area and upper back [Figure - 2] extending to the dorsum of the hands along with involvement of the dorsa of feet upto the ankles. Fissuring was noted over the preauricular area. Characteristic sparing of the periorbital area (patient was using spectacles) and vest covered area was noted. Hair, nail, and mucosae were normal. Skin biopsy was not undertaken.
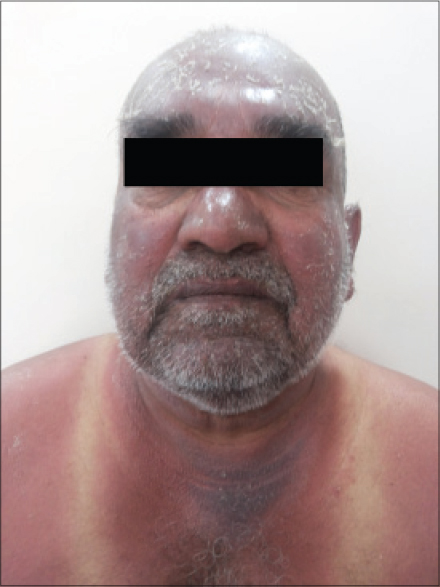 |
| Figure 1: Erythema and scaling involving face, neck, shoulder sparing vest covered area, and periorbital region |
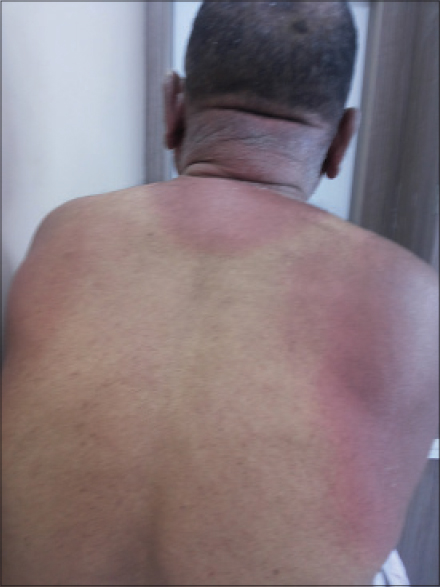 |
| Figure 2: Intense erythema involving upper back, nape of neck, and shoulders sparing the vest covered region |
He had interstitial lung disease and was on treatment with bronchodilators (formetrol, once a day), mucolytics (N-acetylcysteine, 2 teaspoons thrice a day), and pirfenidone for the last 6 months. He had been started on 600 mg/day of pirfenidone 5 months back and the dose was gradually increased to 1200 mg/day. Ten days after escalation of the dose, the patient developed the rash suggesting a dose dependency of the phototoxic reaction. He was managed with oral prednisolone 0.75 mg/kg body weight in a tapering dose along with antihistamines, topical corticosteroids, broad spectrum sunscreens and strict photoprotection.
A diagnosis of drug-induced phototoxic reaction with pirfenidone was made based on clinical examination and history. The differential diagnosis of allergic contact dermatitis, irritant contact dermatitis, and subacute cutaneous lupus erythematosis were excluded on clinical grounds.
Costabel et al. stated that the cutaneous adverse effects of pirfenidone include phototoxic burn-like skin rash on the sun exposed body areas.[2] Skin related photosensitivity reactions were observed in 12.2% and rash was noted in 32.2% of pirfenidone treated patients as reported in the CAPACITY studies.[3] Our patient can be categorized as Grade 3 (erythema with desquamation) which was reported in 0.2% of the drug cohort in the CAPACITY studies. Photosensitivity and rash led to the discontinuation of therapy in approximately 1% of the patient.[3]
The mechanism of pirfenidone-induced photosensitivity is likely to be phototoxic and is related to the drug's ability to absorb ultraviolet A and ultraviolet B. Absorption of ultraviolet light in the skin tissue could result in skin lesions due to generation of reactive oxygen species and lipid peroxidation.[4] Animal studies have shown the use of sunscreens with higher sun protection factor significantly reduce the severity of skin reactions.[5]
The standard approach for preventing and managing skin-related adverse effects include avoiding direct sun exposure, use of broad spectrum sunscreens, physical protection, and avoiding other phototoxic drugs. Costabel et al. have expanded the above guidelines by suggesting behavioral avoidance of indirect sunlight as well as intense artificial light sources, wearing of thick woven cloth and broad brimmed hats and avoiding sun exposure for a few hours following the intake of pirfenidone.[2] The management of the rash includes reduction of the drug dose and discontinuation of the drug in case of persistence of rash for more than 15 days. Slow re-introduction of the drug can be attempted once the symptoms have resolved.
As pirfenidone is also associated with gastrointestinal and neurological side effects, these also need to be carefully monitored. Dose reduction can lessen the gastrointestinal and dermatological side effects.[6]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors have obtained appropriate patient consent for the information published in this article.
| 1. |
Koulelidis A, Zacharis G, Dolios P, Zouvelekis AT. Pirfenidone photosensitivity rash in a patient with idiopathic pulmonary fibrosis. Pneumon 2013;26:92.
[Google Scholar]
|
| 2. |
Costabel U, Bendstrup E, Cottin V, Dewint P, Egan JJ, Ferguson J, et al. Pirfenidone in idiopathic pulmonary fibrosis: Expert panel discussion on the management of drug-related adverse events. Adv Ther 2014;31:375-91.
[Google Scholar]
|
| 3. |
Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011;377:1760-9.
[Google Scholar]
|
| 4. |
Seto Y, Inoue R, Kato M, Yamada S, Onoue S. Photosafety assessments on pirfenidone: Photochemical, photobiological, and pharmacokinetic characterization. J Photochem Photobiol B 2013;120:44-51.
[Google Scholar]
|
| 5. |
Pharmaceuticals and Medical Devices Agency Japan. Pirfenidone Report. Available from: http://www. pmda.go.jp/english/service/pdf/drugs/pirespa_oct2008_e.pdf. [Last accessed on 2014 Mar 18]
[Google Scholar]
|
| 6. |
Jiang C, Huang H, Liu J, Wang Y, Lu Z, Xu Z. Adverse events of pirfenidone for the treatment of pulmonary fibrosis: A meta-analysis of randomized controlled trials. PLoS One 2012;7:e47024.
[Google Scholar]
|
Fulltext Views
5,494
PDF downloads
2,626





