Translate this page into:
Pityriasis rosea - An update
2 Department of Dermatology, NDMVPS Medical College and Research Centre Nashik, Maharashtra, India
3 Department of Dermatology, Mayo Medical School, Mayo Clinic, Rochester, Minnesota, USA
4 Department of Dermatology, University Hospital-Zurich, Zurich, Switzerland
Correspondence Address:
Antonio Chuh
The Bonham Surgery, Shop B5, Ning Yeung Terrace, 78 Bonham Road, Ground Floor
Hong Kong S.A.R.
| How to cite this article: Chuh A, Lee A, Zawar V, Sciallis G, Kempf W. Pityriasis rosea - An update. Indian J Dermatol Venereol Leprol 2005;71:311-315 |
Abstract
Recent controversies on the etiology, diagnosis and treatment have led to increased interest in pityriasis rosea (PR). We review these aspects of the disease. PR is universal. The incidence is around 0.68 per 100 dermatological patients, or 172.2 per 100,000 person-years. The prevalence in people aged between 10 and 29 years is 0.6%. The male to female ratio is around 1: 1.43. Evidence on seasonal variation is conflicting, but there is no evidence that the incidence is dependent on mean air temperature, mean total rainfall, or mean relative humidity. Spatial-temporal and temporal clustering of cases of PR has been reported. The association of PR with human herpesvirus-7 infection is still controversial. Owing to the extreme high sensitivities of sequence-based detection methods such as polymerase chain reaction, novel criteria should be applied to evaluate the evidence. There is no evidence that PR is associated with other viral or bacterial infections. The role of autoimmunity in PR warrants further investigations. Many patients with PR have one or more atypical features. Application of validated diagnostic criteria may be helpful for atypical cases. The efficacy of macrolides, including erythromycin, in PR is still under evaluation. There is no evidence that antiviral agents are effective. The efficacies of ultraviolet radiotherapy and systemic corticosteroids are not well established. In managing a patient with PR, we should concentrate more on how the eruption is affecting the quality of life, i.e. the illness, rather than the extent and severity of the eruption, i.e. the disease.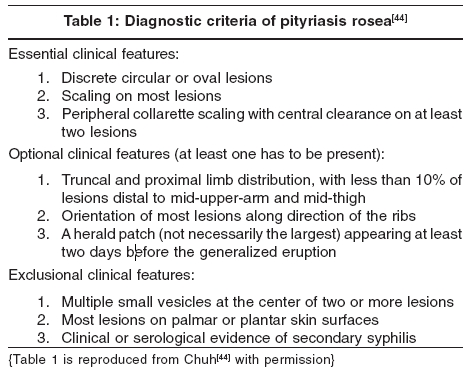

Introduction
Despite active labor for nearly one and a half century by generations of researchers, the etiology of pityriasis rosea (PR) fails to be demystified. Recent controversies on the role of human herpesvirus-7 (HHV-7) in the etiology, the discovery of significant temporal clustering, the establishment of diagnostic criteria, and controversies on the role of macrolides in treatment have led to increased interest in this eruption.
Historical aspects
PR was probably first described by the Edinburgh dermatologist Robert Willan under another terminology in 1798.[1] The macular variety of PR was first named as such by the French dermatologist Camille Melchoir Gibert in 1860.[2] The more usual annular variety was first described by another French dermatologist Pierre-Antoine-Ernest Bazin in 1862.[3]
Jean Baptiste Emile Vidal, another French dermatologist, described pityriasis circinι et marginι in 1882.[3] It has fewer and larger lesions, often localized at the axillae or groins, and runs a longer course.[4] The herald patch was first described by a French dermatologist Louis-Anne-Jean Brocq in 1887.[1]
Nomenclature
In ICD-10, pityriasis rosea is coded L42X00. Pityriasis rosea due to a drug is considered a distinct condition. Pityriasis circinata et marginata of Vidal is considered a synonym of PR.
Epidemiology
PR is universal. Early epidemiology reports were from France and the United Kingdom[2] and more recent ones from the United Kingdom, [5],[6],[7],[8], Uganda,[9] Nigeria,[10] United States,[11] Brasil,[12] Sudan,[13] Lagos,[14] Singapore,[15],[16] Turkey,[17] Kuwait,[18] Burkina Faso,[19] and Hong Kong.[20]
These studies reported a range of incidence from 0.39[12] to 4.80[14] per 100 dermatological patients. Analyzing all patients in nine recent studies, [9],[10],[11],[12],[13],[14],[15],[16],[17],[18], we found an overall incidence of 0.68 per 100 dermatological patients. The community-based incidence of PR was reported to be 172.2 per 100,000 person-years.[11] The prevalence was reported to be 0.6% for young people aged between 10 and 29 years.[19]
Most patients are between the age of 10 and 35 years.[22] Analyzing 15 recent epidemiology studies, [5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20], we found that out of a total of 3850 patients, 1584 were males and 2266 were females. The overall male to female ratio is thus 1:1.43.
Conflicting results were reported for seasonal variation in PR. A higher incidence in the colder months was reported by investigators in England,[8] United States,[11] and Sudan.[13] A higher incidence in the early part of the rainy season was reported in Lagos.[14] A bimodal distribution was reported in Brasil[12] and in Singapore.[15] Other studies reported no seasonal variation.[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20], The incidence of PR has been reported to be independent of the monthly mean air temperature, mean total rainfall and mean relative humidity in Hong Kong.[20]
Clusters of cases have been frequently reported.[7] Spatial-temporal[8] and temporal[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20],clustering has been reported, offering epidemiological support for an infectious etiology.
Etiology
PR has long been suspected to have an infectious, mainly viral, etiology because of a distinct clinical course akin to those of viral exanthems. That most sufferers will not have a relapse in their lifetime is suggestive of immunity against the virus upon the first attack. And, as discussed in the preceding paragraph, clustering of cases[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20], also indirectly supports an infective etiology. There have also been reports associating PR with a history of respiratory tract infections,[23] unfavorable social and economic backgrounds,[19] and contact with patients with PR.[24] PR is not associated with atopy or an atopic tendency.[23]
Recent controversy is centered on the association of PR with HHV-7 infection. Investigators have reported positive[25],[26],[27],[28],[29],[30],[31], and negative[32],[33] results. A systematic review of the evidence has been published.[34] Association is best considered controversial. Owing to the extreme high sensitivities of sequence-based laboratory detection methods, a logical step would be to apply standard criteria to evaluate the strength of association. There is convincing evidence that PR is not associated with cytomegalovirus,[26],[27],[28],[29],[30],[31],[32],[33],[34],[35],Epstein-Barr virus,[35] parvovirus B19,[35] picornavirus,[36] influenza virus,[37] parainfluenza virus,[37] Chlamydia pneumoniae ,[38] C. trachomatis ,[38] Legionella longbeachae ,[38] L. micdadei ,[38] L. pneumophila ,[38] and Mycoplasma pneumoniae[37],[38] infections.
A study reported that 28% of patients with PR have T lymphocytotoxic antibodies.[39] Thes autoantibodies can be detected in 82% of patients with systemic lupus erythematosus. Patients with PR are significantly more likely to have anti-nuclear antibodies detectable.[40] The role of autoimmunity in PR warrants further investigations.
Clinical presentation and diagnosis The incidence of the herald patch varies to a great extent, from 40-76% in various studies. [5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19], Its absence does not exclude the diagnosis. The range of the interval between the primary and secondary eruptions is also wide, from 2 to 84 days. It is predominantly around 7 to 14 days. Recognition of the peripheral collarette-scaling pattern was reported to be enhanced by epiluminescence dermatoscopy.[41] Characteristic orientation of the secondary eruption is best described as "along lines of skin cleavages". "Christmas-tree", "inverted Christmas-tree", "fur tree", and "parallel to the ribs" are imprecise and probably obsolete descriptions of the orientation.[42]
African patients have a more extensive rash,[9],[10] with more frequent involvement of the face and scalp.[9] The rash is not erythematous.[13] Post-inflammatory hypopigmentation is a cosmetic concern for dark-skinned patients.
The incidence of patients with atypical clinical features is difficult to quantify, but is likely to be high.[43] Rash morphology may be atypical, as vesicular, purpuric or hemorrhagic, and urticarial variants. PR with enormous plaques is known as pityriasis rosea gigantea of Darier. The other extreme is very small lesions in papular PR. The face, axillae, and groins are predominantly involved in PR inversus. The shoulders and hips are predominantly affected in limb-girdle PR. Involvement of the face, scalp, hands, and feet is not rare in PR. Involvement of mucous membranes such as the oral cavity has been reported. The severity may also be atypical. Patients with severe pruritus, pain, and a burning sensation can be said to have PR irritata.
A set of diagnostic criteria has been devised and validated for PR [Table - 1],[44] but its reliability and applicability in other ethnic groups remain to be ascertained.
Treatment
Antibiotics such as erythromycin and other macrolides are being used to treat PR, probably on the basis of a pseudo-randomized study using erythromycin.[45] We believe that the use of macrolides is best considered experimental, and should not be adopted into routine clinical practice until results of further studies are published. Even if macrolides are finally proven to be effective in modifying the course of PR, this does not substantiate that PR is caused by a bacterial rather than a viral infection. Macrolides have anti-inflammatory and immunomodulating effects that might affect the course of PR or other cutaneous eruptions independent of their antibacterial properties.
Acyclovir has been recently advocated in an indexed article for the treatment of PR.[46] However, it is too premature to adopt the use of anti-viral agents into routine clinical practice. The association of PR with any virus, including HHV-7, is not yet firmly established. Even if PR is caused by primary infection or reactivation of HHV-7, acyclovir is a poor choice for antiviral therapy[47] since it has little to no action against HHV-7 in vitro. HHV-7 lacks the thymidine kinase gene while the action of acyclovir is thymidine kinase-dependent.
The use of ultraviolet radiotherapy is controversial, as studies have reported conflicting results. We understand that some dermatologists are using systemic corticosteroids for patients with particularly recalcitrant PR.[16] A Cochrane review is in progress on the interventions in PR.[48] It might shed more light on the issue.
To many patients, PR means nothing more than a weird dermatological diagnosis. Many do not experience any pruritus despite an extensive eruption. For adults with PR, the effects on the quality of life are independent of the rash severity.[49] For children with PR, the quality of life is only affected to a very limited extent.[50] However, most clinical trials adopt physician-rated outcome measures such as extensiveness of rash, rather than patient-rated outcome measures such as pruritus or quality of life measurements. These trials might document the efficacy of the intervention on the disease as experienced by the doctor, but not the illness as experienced by the patient. We shall leave the reader to decide which of these two is the more pertinent issue.
| 1. |
Weiss L. Pityriasis rosea - an erythematous eruption of internal origin. JAMA 1903;41:20-8.
[Google Scholar]
|
| 2. |
Percival GH. Pityriasis rosea. Br J Dermatol 1932;44:241-53.
[Google Scholar]
|
| 3. |
Klauder JV. Pityriasis rosea with particular reference to its unusual manifestations. JAMA 1924;82:178-83.
[Google Scholar]
|
| 4. |
Sarkany I, Hare PJ. Pityriasis rotunda (pityriasis circinata). Br J Dermatol 1964;76:223-7.
[Google Scholar]
|
| 5. |
Abercrombie GF. Pityriasis rosea. Proc R Soc Med 1962;55:556-7.
[Google Scholar]
|
| 6. |
Cohen EL. Pityriasis rosea. Br J Dermatol 1967;79:533-7.
[Google Scholar]
|
| 7. |
Abercrombie GF. Pityriasis rosea 1964-65. J R Coll Gen Pract 1968;16:268-74.
[Google Scholar]
|
| 8. |
Messenger AG, Knox EG, Summerly R, Muston HL, Ilderton E. Case clustering in pityriasis rosea: support for role of an infective agent. Br Med J 1982;284:371-3.
[Google Scholar]
|
| 9. |
Vollum DI. Pityriasis rosea in the African. Trans St Johns Hosp Dermatol Soc 1973;59:269-71.
[Google Scholar]
|
| 10. |
Jacyk WK. Pityriasis rosea in Nigerians. Int J Dermatol 1980;19:397-9.
[Google Scholar]
|
| 11. |
Chuang TY, Ilstrup DM, Perry HO, Kurland LT. Pityriasis rosea in Rochester, Minnesota, 1969 to 1978. J Am Acad Dermatol 1982;7:80-9.
[Google Scholar]
|
| 12. |
de Souza Sittart JA, Tayah M, Soares Z. Incidence pityriasis rosea of Gibert in the Dermatology Service of the Hospital do Servidor Publico in the state of Sao Paulo. Med Cutan Ibero Lat Am 1984;12:336-8.
[Google Scholar]
|
| 13. |
Ahmed MA. Pityriasis rosea in the Sudan. Int J Dermatol 1986;25:184-5.
[Google Scholar]
|
| 14. |
Olumide Y. Pityriasis rosea in Lagos. Int J Dermatol 1987;26:234-6.
[Google Scholar]
|
| 15. |
Cheong WK, Wong KS. An epidemiological study of pityriasis rosea in Middle Road Hospital. Singapore Med J 1989;30:60-2.
[Google Scholar]
|
| 16. |
Tay YK, Goh CL. One-year review of pityriasis rosea at the National Skin Centre, Singapore. Ann Acad Med Singapore 1999;28:829-31.
[Google Scholar]
|
| 17. |
Harman M, Aytekin S, Akdeniz S, Inaloz HS. An epidemiological study of pityriasis rosea in the Eastern Anatolia. Eur J Epidemiol 1998;14:495-7.
[Google Scholar]
|
| 18. |
Nanda A, Al-Hasawi F, Alsaleh QA. A prospective survey of pediatric dermatology clinic patients in Kuwait: an analysis of 10,000 cases. Pediatr Dermatol 1999;16:6-11.
[Google Scholar]
|
| 19. |
Traore A, Korsaga-Some N, Niamba P, Barro F, Sanou I, Drabo YJ. Pityriasis rosea in secondary schools in Ouagadougou, Burkina Faso. Ann Dermatol Venereol 2001;128:605-9.
[Google Scholar]
|
| 20. |
Chuh A, Lee A, Molinari N. Case clustering in pityriasis rosea - a multi-center epidemiological study in primary care settings in Hong Kong. Arch Dermatol 2003;139:489-93.
[Google Scholar]
|
| 21. |
Giam YC. Skin diseases in children in Singapore. Ann Acad Med Singapore 1988;17:569-72.
[Google Scholar]
|
| 22. |
Truhan AP. Pityriasis rosea. Am Fam Physician 1984;29:193-6.
[Google Scholar]
|
| 23. |
Chuang TY, Perry HO, Ilstrup DM, Kurland LT. Recent upper respiratory tract infection and pityriasis rosea: a case-control study of 249 matched pairs. Br J Dermatol 1983;108:587-91.
[Google Scholar]
|
| 24. |
McPherson A, McPherson K, Ryan T. Is pityriasis rosea an infectious disease? Lancet 1980;2:1077.
[Google Scholar]
|
| 25. |
Drago F, Ranieri E, Malaguti F, Losi E, Rebora A. Human herpesvirus 7 in pityriasis rosea. Lancet 1997;349:1367-8.
[Google Scholar]
|
| 26. |
Drago F, Ranieri E, Malaguti F, Battifoglio ML, Losi E, Rebora A. Human herpesvirus 7 in patients with pityriasis rosea. Electron microscopy investigations and polymerase chain reaction in mononuclear cells, plasma and skin. Dermatology 1997;195:374-8.
[Google Scholar]
|
| 27. |
Watanabe T, Sugaya M, Nakamura K, Tamaki K. Human herpesvirus 7 and pityriasis rosea. (correspondence) J Invest Dermatol 1999;113:288-9.
[Google Scholar]
|
| 28. |
Drago F, Malaguti F, Ranieri E, Losi E, Rebora A. Human herpes virus-like particles in pityriasis rosea lesions: an electron microscopy study. J Cutan Pathol 2002;29:359-61.
[Google Scholar]
|
| 29. |
Watanabe T, Kawamura T, Jacob SE, Aquilino EA, Orenstein JM, Black JB, et al. Pityriasis rosea is associated with systemic active infection with both human herpesvirus-7 and human herpesvirus-6. J Invest Dermatol 2002;119:793-7.
[Google Scholar]
|
| 30. |
Vαg T, Sonkoly E, Kemeny B, Kαrpαti S, Horvath A, Ongrαdi J. [Studies on the novel association of human herpesvirus-7 with skin diseases.] Orv Hetil 2003;144:1623-9.
[Google Scholar]
|
| 31. |
Vαg T, Sonkoly E, Kαrpαti S, Kemιny B, Ongrαdi J. Avidity of antibodies to human herpesvirus 7 suggests primary infection in young adults with pityriasis rosea. J Eur Acad Dermatol Venereol 2004;18:738-40.
[Google Scholar]
|
| 32. |
Kempf W, Adams V, Kleinhans M, Burg G, Panizzon RG, Campadelli-Fiume G, et al . Pityriasis rosea is not associated with human herpesvirus 7. Arch Dermatol 1999;135:1070-2.
[Google Scholar]
|
| 33. |
Chuh AA, Chiu SS, Peiris JS. Human herpesvirus 6 and 7 DNA in peripheral blood leukocytes and plasma in patients with pityriasis rosea by polymerase chain reaction - a prospective case control study. Acta Derm Venereol 2001;81:289-90.
[Google Scholar]
|
| 34. |
Chuh AA, Chan H, Zawar V. Is human herpesvirus 7 infection the causative agent of pityriasis rosea? - a critical review. Int J Dermatol 2004;43:870-5.
[Google Scholar]
|
| 35. |
Chuh AA. The association of pityriasis rosea with cytomegalovirus, Epstein-Barr virus and parvovirus B19 infections - a prospective case control study by polymerase chain reaction and serology. Eur J Dermatol 2003;13:25-8.
[Google Scholar]
|
| 36. |
Aractingi S, Morinet F, Mokni M, Tieng V, Flageul B, Fermand JP, et al . Absence of picornavirus genome in pityriasis rosea. Arch Dermatol Res 1996;289:60-1.
[Google Scholar]
|
| 37. |
Hudson LD, Adelman S, Lewis CW. Pityriasis rosea. Viral complement fixation studies. J Am Acad Dermatol 1981;4:544-6.
[Google Scholar]
|
| 38. |
Chuh AA, Chan HHL. Prospective case-control study of chlamydia, legionella and mycoplasma infections in patients with pityriasis rosea. Eur J Dermatol 2002;12:170-3.
[Google Scholar]
|
| 39. |
Hosokawa H, Horio S, Takiuchi Y, Maruyama N, Asada Y. Naturally occurring T lymphocytotoxic antibody in viral and related skin diseases. Acta Derm Venereol 1984;64:275-80.
[Google Scholar]
|
| 40. |
Chuh AA. A prospective study of autoimmune markers in patients with pityriasis rosea. Clin Exp Dermatol 2003;28:449-50.
[Google Scholar]
|
| 41. |
Chuh AA. Collarette scaling in pityriasis rosea demonstrated by digital epiluminescence dermatoscopy. Australas J Dermatol 2001;42:288-90.
[Google Scholar]
|
| 42. |
Chuh AA. Rash orientation in pityriasis rosea - a qualitative study. Eur J Dermatol 2002;12:253-6.
[Google Scholar]
|
| 43. |
Chuh A, Zawar V, Lee A. Atypical presentations of pityriasis rosea: case presentations. J Eur Acad Dermatol Venereol 2005;19:120-6.
[Google Scholar]
|
| 44. |
Chuh AA. Diagnostic criteria for pityriasis rosea - a prospective case control study for assessment of validity. J Eur Acad Dermatol Venereol 2003;17:101-3.
[Google Scholar]
|
| 45. |
Sharma PK, Yadav TP, Gautam RK, Taneja N, Satyanarayana L. Erythromycin in pityriasis rosea: A double-blind, placebo-controlled clinical trial. J Am Acad Dermatol 2000;42:241-4.
[Google Scholar]
|
| 46. |
Castanedo-Cazares JP, Lepe V, Moncada B. Should we still use phototherapy for pityriasis rosea? Photodermatol Photoimmunol Photomed 2003;19:160-1.
[Google Scholar]
|
| 47. |
Chuh AA. Narrow band UVB phototherapy and oral acyclovir for pityriasis rosea. Photodermatol Photoimmunol Photomed 2004;20:64-5.
[Google Scholar]
|
| 48. |
Chuh A, Dofitas B, Comisel G, Reveiz L, Morar N, Sharma V, et al. Interventions in pityriasis rosea - a protocol. Cochrane Database Syst Rev 2005 (in press).
[Google Scholar]
|
| 49. |
Chuh AA, Chan HH. Effect on quality of life in patients with pityriasis rosea - Is it associated with rash severity? Int J Dermatol 2005;44:372-7.
[Google Scholar]
|
| 50. |
Chuh AA. Quality of life in children with pityriasis rosea - a prospective case control study. Pediatr Dermatol 2003;20:474-8.
[Google Scholar]
|
Fulltext Views
14,685
PDF downloads
3,330





