Translate this page into:
Position paper on mesotherapy
2 Venkat Charmalaya, Centre for Advanced Dermatology, Bangalore, India
Correspondence Address:
Rashmi Sarkar
Department of Dermatology, Maulana Azad Medical College, New Delhi - 110 002
India
| How to cite this article: Sarkar R, Garg VK, Mysore V. Position paper on mesotherapy. Indian J Dermatol Venereol Leprol 2011;77:232-237 |
Abstract
Mesotherapy is a controversial cosmetic procedure which has received publicity among the lay people, in the internet and in the media. It refers to minimally invasive techniques which consist of the use of intra- or subcutaneous injections containing liquid mixture of compounds (pharmaceutical and homeopathic medications, plant extracts, vitamins and other ingredients) to treat local medical and cosmetic conditions. This position paper has examined the available evidence and finds that acceptable scientific evidence for its effectiveness and safety is lacking. IADVL taskforce, therefore would like to state that the use of this technique remains controversial at present. Further research and well-designed controlled scientific studies are required to substantiate the claims of benefit of this mode of therapy.Introduction
The technique involving direct injections of medications into the skin was first described by a French physician, Dr. Michel Pistor in 1952 when he administered procaine intravenously to an asthmatic patient, which had limited impact on his airway disease but instead, improved his hearing. [1] It was later recognized as the original application of mesotherapy, which included improvement of joint pain, eczema and tinnitus. [2],[3] Pistor subsequently coined the term ′mesotherapy" (there is another view that the French press coined the term,"mesotherapy") which meant as "treatment of the mesoderm, one of the three primary germ layers which later develops into connective tissue, muscle and the circulating system". [4] Therefore, though originally developed to treat vascular, lymphatic and hematological conditions, due to an increasing demand for noninvasive cosmetological procedures, mesotherapy has attracted a lot of interest from physicians and the general public as a treatment modality for cellulite treatment, lipolysis or "fat dissolving "and body contouring. [5],[6],[7],[8]
Despite it being available for over 50 years and the huge publicity and attention received on the internet, definite evidence for its efficacy is lacking and the claims are not always based on well conducted clinical trials. Anecdotal reports are often touted as evidence and heavy advertisements in media sustain its popularity. Federal drug administration (FDA), USA has not approved this method of treatment. Some of the compounds used in mesotherapy have been approved by FDA for human use, but for a different purpose or indication. Many dermatologists and other physicians practice this technique and patients often seek the opinions of dermatologists about the efficacy of this technique. This paper conducts a review of the subject, examines the available evidence and formulates the official policy statement on behalf of IADVL.
Definition
Mesotherapy, refers to a variety of minimally invasive techniques which consist of the use of intra- or subcutaneous liquid injections containing mixture of compounds to treat local medical and cosmetic conditions. The injections could include hormones, enzymes, pharmaceuticals, nutrients, homeopathic agents, detergents and other substances which are injected in between the dermis and the skin known as mesoderm. [9],[10]
Reported indications and possible rationale
Mesotherapy was originally invented for pain relief; however, its cosmetic applications including fat and cellulite removal and facial rejuvenation, have received attention. A distinction has been made between mesotherapy (injections in to mesoderm to produce effects on mesoderm) and injection lipolysis (also called lipodissolve-a method of treating localized adipose tissue with subcutaneous injections of deoxycholate either alone or in combination with phosphatidlycholine). [4],[11],[12],[13] Phosphatidylcholine and deoxycholate injections are used subcutaneously for their local effect only, for treating localized deposits of adipose tissue in contrast to mesotherapy, which as per definition, is said to affect " mesoderm". Opinion has been expressed, therefore, that injections of phosphatidyl choline and deoxycholate are local injection therapies and not "Mesotherapy". [4] This distinction assumes importance in view of the fact while there have been several publications in indexed journals to support the efficacy of injection lipolysis, very little published data exists to support the role of mesotherapy.
[Table - 1] mentions the main dermatological and other medical indications purported to benefit from mesotherapy. It is further clarified that this list is only a list of reported indications for which claims of benefit have been made. The indications for which published data exists is discussed below.
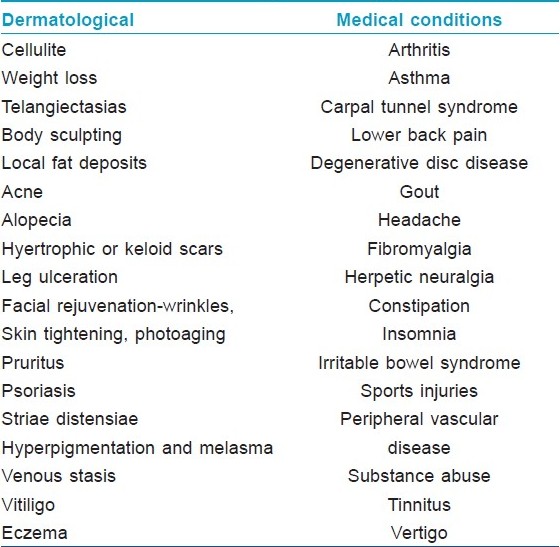
Cellulite (Level D) [8],[14],[15],[16] Cellulite, a skin surface change that is common in women, is a much debated condition whose etiology is unknown and remains elusive to treatment. Despite the lack of evidence to support efficacy, treatment options, including mesotherapy continue to proliferate. Caruso et al, evaluated the lipolytic potentials of solutions used in the practice of mesotherapy to stimulate lipolysis, cause local fat reduction and treating cellulite. These were tested in a human fat cell assay using the induction of glycerol generation as a measure of lipolysis. Isoproterenol (P<0.002), aminophylline (P<0.00004) and yohimbine (P<0.001) stimulated lipolysis compared to the buffer control. It was observed that isoproterenol, aminophylline, yohimbine and melilotus stimulate lipolysis alone, and lipolysis is further enhanced by combining lipolytic stimulators in mesotheray solutions. It was also observed that lidocaine is antilipolytic and should be removed from mesotherapy solutions designed for local fat reduction. [16] A review of the subject concluded that "Until further studies are performed, patients considering mesotherapy for cellulite must be aware that the substances currently being injected to treat this cosmetically disturbing, but medically benign, condition have not been thoroughly evaluated for safety or efficacy." [8]
Weight loss and body sculpting (Level C) [7],[17],[18] A randomized, prospective case-controlled study over a 12-week period by Park et al, to study the effect of a mixed solution (i.e. aminophylline, buflomedil and lidocaine) by injecting it into the superficial dermis of the medial aspect of the thigh weekly with the other thigh acting as a control, showed no significant loss of thigh girth on the treated side as compared to the non-treated thigh as measured by computed tomographic scanning. The study indicated poor patient satisfaction and demonstrated that mesotherapy is not an effective alternative treatment modality for body contouring. [18]
Local fat deposits - xanthelasma, lipoma, submental fat reduction (Level C), [19],[20],[21],[22]In an open-label clinical trial, Hexsel et al, treated 213 patients with HIV lipodystrophy, lipomas, buffalo hump, on the chin, trunk and extremities with 0.2 ml phosphatidylcholine placed every 1.5-2.0 cm into the lipomas every 15 days upto five treatments. Thirteen non-HIV patients had serum laboratory testing before, 48 hours and 2 weeks post-treatment after two treatments. It was observed that vast majority of patients had reduction of fat thickness after up to five treatments. All buffalo hump patients reported improvement. There were no significant alterations in hepatic and lipid profiles. [21] In another study, use of phosphatidylcholine vs. phosphatidylcholine plus organic silicum was effective in reducing submental fat in 12 patients. The rate and degree of reduction was not significantly different after three treatment sessions. Adverse reactions were few, mild and transitory. Hence both regimens appeared safe, efficacious and cost-effective. [22] These studies suggest that phosphatidylcholine injection, which are per se not mesotherapy by definition may have a role in treating localized fat deposits and further studies are necessary to establish its safety and efficacy.
Mesolift or facial rejuvenation - (Level C) [9],[23] Amin et al, conducted four sessions of mesotherapy involving multiple injections of a multivitamin and hyaluronic acid solution, at four monthly intervals for facial rejuvenation on 10 subjects and the results were evaluated photographically and histopathologically. The study revealed no significant clinical or histological changes due to mesotherapy with multivitamin and hyaluronic acid solutions. [9] However, in another study, Lacarrubba et al, [23] evaluated the effects of mesotherapy with multiple injections of hyaluronic acid in 20 women with physical signs of moderate skin photoaging with subepidermal low-echogenic band (SLEB) on ultrasonography of skin. This study suggested that mesotherapy with hyaluronic acid maybe an effective treatment for photoaging, as there was a statistically significant (P<0.001) increase in SLEB echogenecity in 15/19 patients who completed the study. [23] The study recommended that further follow-up investigations on larger series of patients are necessary to further evaluate the safety, effectiveness and duration of effect of this possible therapeutic approach to skin photoaging.
While mesotherapy has been claimed to be beneficial in several other conditions such as alopecia, melasma as listed in [Table - 1], there is no published paper supporting their use or documenting their efficacy.
Contraindications to therapy [5],[10]
The contraindications to mesotherapy are:
- Pregnant and lactating females
- Insulin dependent diabetes mellitus
- History of bleeding disorders
- History of strokes
- History of thromboembolic phenomena
- Patients on medication for cardiac arrythmias, aspirin, warfarin, heparin, etc.
- History of recent cancer
- Severe heart disease
- Renal disease
- Any severe chronic systemic disease
Products commonly used for mesotherapy
A wide variety of agents have been used in mesotherapy, including vitamins, herbal agents, homeopathic medications, and others. These include:
- Dissolving fat - Phosphatidyl choline [12],[13],[15],[19] (Level B); aminophylline [16],[18] (Level C), theophyllline, caffeine, ephedrine, calcium pyruvate, carnitine (Level D), organic silicium [23] (Level C).
- Collagen rejuvenation-tretinoin, organic silicium (No evidence)
- Collagen remodelling-Hyaluronidase (level C), collagenase
- Skin hydration, tightening, exfoliation-Hyaluronic acid [23] (Level C), prochlorperazine, dimethylaminoethanol (DMAE), silica, glycolic acid (Level D). [24]
- Agents that act as antioxidants and decrease skin pigmentation-Vitamin C (hyperpigmentaion and melasma), glutathione, glycolic acid, pyruvate, biotin (alopecia), pantothenic acid, vitamin E and A, minerals like selenium, zinc, copper, magnesium, chromium, a-lipoic acid and melatonin (No specific evidence)
- Hair growth stimulation - Finasteride, minoxidil, buflomedil (No specific evidence)
- Improvement of collagen and elastic synthesis and cytokines for cellular stimulation - CRP 1000 and copper peptide.
- Circulatory stimulants - pentoxyphylline, coumarin, arnica, ginko biloba, melilotus, yohimbine. (No specific evidence)
- Antiinflammatory - Piroxicam, ketorolac.
- Calcium deposition removal - calcitonin
- Immune stimulation - Vaccine, interferon
- Antibiotics and chemotherapeutic agents - Metronidazole
- Analgesics, muscle relaxants and tranquillizers - diazepam, baclofen and orphenadrine are muscular analgesics
- Nausea reduction - Prochlorperazine
- Local anaesthetics-Procaine, prilocaine and lidocaine [16] (Level C)
- Others-T3-T4 thyroid, isoproterenol, pentoxifylline, L-carnitine, L-arginine, co-enzymes, cofactors, dimethylethanolamine, C-adenosine monophosphate, multiple vitamins [22] (Level C), trace mineral elements, carbon dioxide.
It needs to be emphasized here that there are no standardized ingredients or dosages for these ingredients and these are used in arbitrary cocktail formulations, based on their known theoretical pharmacological actions. While some products have been used orally or intravenously for several years, there is little knowledge about pharmacodynamics and kinetics of the products when injected in to skin. These products are not subject to rigorous regulations of federal drug authorities. There is no data regarding interaction, effectiveness and safety of mixing different ingredients. Likewise, there are variations in techniques of administrations, instruments used, frequency of administration, treatment etc. In view of this, these aspects are not discussed here.
Complications
Local
In contrast to the claims of mesotherapy being a safe, minimally invasive procedure, several side effects have been reported. It is also interesting to note that in contrast to the lack of data on its efficacy, there are a number of published studies about its complications, as follows:
- Bruising and edema due to the chemicals used in mesotherapy [10],[25]
- Skin necrosis [25],[26]
- Atypical mycobacterial infections [27]
- Facial and scalp ulcers and scarring [28],[29]
- Allergic reactions due to various chemicals [25],[30]
- Atrophy and lipodystrophy [25]
- Lichenoid eruption [31]
- Postinflammatory hyperpigmentation [4]
- Nodularity and irregularity after lipolysis
- Rare: Koebnerization in psoriasis, [25] localized urticaria pigmentosa [32] granuloma annulare, [33] noninfectious granulomatous panniculitis, [34] alopecia [35]
- Allergic reactions
- Vagal syndromes
- Lipothymia
- Infections (HIV, hepatitis etc)
- Liver toxicity and demyelination of nerves due to large doses of phosphatidylcholine
These reports contradict the offrepeated claims of safety of mesotherapy and emphasize the necessity for proper safety precautions and patient counseling during the treatment. Thus, the claims of safety and simplicity of administration can be misleading and lead to self treatments by patients themselves, as exemplified by a reported case of self administration of lipase obtained on the internet. [36]
Position Statement of IADVL Taskforce on the use of Mesotherapy in Dermatosurgery Practice
Although mesotherapy is a well-advertised therapeutic modality on the internet and media, and is practiced in Europe and South America, data on its safety and efficacy in cosmetic conditions are limited. There are currently no adequate, peer-reviewed clinical trials critically evaluating the efficacy of mesotherapy for dermatological and aesthetic indications. While, there are a few peer reviewed publications on the efficacy of subcutaneous injections of phosphatidylcholine in treating localized collections of fat (which as stated earlier is different from mesotherapy), these are few and of low eveidence levels. Hence proper controlled data and more evidence is needed before any recommendations about its usage can be made.
At present there is insufficient data evaluating the safety of the technique and pharmacology, of the combination of herbal and allopathic medicines used. The mechanism of action of many of the products is either doubtful or unknown and there are no clear cut guidelines on the dosage and efficacy of the products. The CDC has recommended that "providers should adhere to recommend standard precautions, follow safe-injection practices with appropriate aseptic techniques, and inject only FDA approved products that are prepared following guidelines to ensure sterility as described in the FDA′s good manufacturing practices." [37] Further, mesotherapy is not entirely a safe technique as is publicized and can give rise to various complications, as reported earlier, some of which are particularly significant in this era of HIV.
Therefore, although it appears to be a simple, easy and financially attractive therapeutic option in cosmetology, the use of this technique is controversial. [38] It is important to understand that the technique has been in practice for over 50 years and yet, little evidence in term of controlled data have been published by users of the technique. Continued research and well designed controlled scientific studies are required to substantiate the claims of effectiveness of these products and to formulate guidelines and recommendations regarding it use for aesthetic applications. It is relevant to note that in a recent publication of guidelines on aesthetic practices in Singapore, mesotherapy is listed as a List B procedure (which indicates procedures with low or very low evidence or local medical expert consensus that procedure is neither well-established nor acceptable.) [39] Such List B procedures include, in addition to mesotherapy, other procedures such as carboxytherapy, skin whitening injections, stem cell activator protein for skin rejuvenation, negative pressure procedures (e.g., Vacustyler TM ), mechanized massage (e.g., Slidestyler TM , endermologie for cellulite treatment) etc. [40]
In view of these, IADVL taskforce does not recommend the routine use of the technique and suggests that scientifically driven prospective double blind controlled studies must be undertaken at many centres to evaluate the safety and efficacy of this therapy. It should also be understood that the final responsibility in this regard lies entirely with the treating physician, if he/she chooses to use the technique, who should therefore exert utmost care to prevent medicolegal situations and take all precautions before administering the treatment.
Acknowledgements
Drs. Hema Jerajani, Shyam Verma and Koushik Lahiri for their review and advice regarding the draft of this article.
| 1. |
Pistor M. What is mesotherapy? Chir Dent Fr 1976;46:59-60.
[Google Scholar]
|
| 2. |
Brown SA. The science of mesotherapy: Chemical anarchy. Aesthetic Surg J 2006;26:95-8.
[Google Scholar]
|
| 3. |
Dalloz-Bourguignon A. A new therapy against pain, mesotherapy. J Belge Med Phys Rehabil 1979;2:230-4.
[Google Scholar]
|
| 4. |
Rotunda AM, Kolodney MS. Mesotherapy and Phosphatidyl choline injections: Historical clarification and review. Dermatol Surg 2006;32:456-80.
[Google Scholar]
|
| 5. |
American Society for Aesthetic Plastic Surgery. National plastic surgery statistics: Cosmetic and reconstruction procedure trends. New York: American Society for Aesthetic Plastic Surgery. Available from: http://www.plasticsurgery.org/public_education/statistical_trends.cf [last accessed on 2003].
[Google Scholar]
|
| 6. |
Bryant R. Controversial mesotherapy: Could it be the next Botox? Derm Times 2004;25:1.
[Google Scholar]
|
| 7. |
Matarasso A, Pfeifer TM. Mesotherapy for body contouring. Plastic Reconstr Surg 2005;115:1425.
[Google Scholar]
|
| 8. |
Rotunda AM, Avram MM, Avram A. Cellulite: Is there a role for injectable? J Cosmet Laser Ther 2005;7:147-54.
[Google Scholar]
|
| 9. |
Amin SP, Phelps RG, Goldberg DJ. Mesotherapy for facial skin rejuvenation: A clinical, histologic and election microscopic evaluation. Dermatol Surg 2006;32:1467-72.
[Google Scholar]
|
| 10. |
Vedamurthy M. Mesotherapy. Indian J Dermatol Venereol Leprol 2007;73:60-2.
[Google Scholar]
|
| 11. |
Duncan DI, Hasengschwandtner F. Lipodissolve for subcutaneous fat reduction and skin retraction. Aesthetic Surg J 2005;25:530-43.
[Google Scholar]
|
| 12. |
Ablon G, Rotunda AM. Treatment of lower eyelid pads using phosphatidyl choline: Clinical trial and review. Dermatol Surg 2004;30:422-7.
[Google Scholar]
|
| 13. |
Rotunda AM, Suzuki H, Moy RL, Kolodney MS. Detergent effects of sodium deoxycholate are a major feature of an injectable phosphatidyl choline formulation used for localized fat dissolution. Dermatol Surg 2004;30:1001-8.
[Google Scholar]
|
| 14. |
The American Society of Aesthetic Plastic Surgery. Cellulite: An update. New York: American Society for Aesthetic Plastic Surgery. Available from: http://www.cbsnews.com/stories/2003/04/28/48hrs/main551361.shtml [last accessed on 2004].
[Google Scholar]
|
| 15. |
Rose PT, Morgan M. Histological changes associated with mesotherpy for fat dissolution. J Cosmet Laser Ther 2005;7:17-9.
[Google Scholar]
|
| 16. |
Caruso MK, Roberts AT, Bissoon L, Self KS, Guillot TS, Greenway FL. An evaluation of mesotherapy solutions for inducing lipolysis and treating cellulite. J Plast Reconstr Aesthet Surg 2008;61:1321-4.
[Google Scholar]
|
| 17. |
Matarasso A, Pfeifer TM. Plastic Surgery Educational Foundation DATA committee: Mesotherapy for body contouring. Plast Reconstr Surg 2005;115:1420-4.
[Google Scholar]
|
| 18. |
Park SH, Kim DW, Lee MA, Yoo Sc, Rhee SC, Koo SH, et al. Effectiveness of mesotherapy on body contouring. Plast Reconstr Surg 2008;121th : 179e-85e.
[Google Scholar]
|
| 19. |
Maggie S. Treatment of xanthelasma with phosphatidyl choline. 5 th International meeting of mesotherapy, Paris, France, 1988.
[Google Scholar]
|
| 20. |
Bechara FG, Sand M, Sand D. Ultrasound controlled injection lipolysis of lipomas with phosphatidyl choline in patients with familial multiple lipomatosis. American Society for Dermatologic Surgery. American College of Mohs micrographic surgery and cutaneous oncology. Combined annual meeting, Atlanta, GA, October 27-30, 2005.
[Google Scholar]
|
| 21. |
Hexsel D, Serra M, Mazzuco R, Dal Forno J, Zechmeister D. Phosphatidylcholine in the treatment of localized fat. J Drugs Dermatol 2003;2:511-8.
[Google Scholar]
|
| 22. |
Co CA, Abad-Casintaham MF, Espinoza-Thaebtharm A. Submental fat reduction by mesotherapy using phosphatidylcholine alone vs.phosphatidylcholine and organic silicum: A pilot study. J Cosmet Dermatol 2007;6:250-7.
[Google Scholar]
|
| 23. |
Lacarruba F, Tedeschi A, Nardone B, Micali G. Mesotherapy for skin rejuvenation: Assessment of the subepidermal low-echogenic band by ultrasound evaluation with cross-sectional B-mode scanning. Dermatol Ther 2008;21:S1-5.
[Google Scholar]
|
| 24. |
Available from: http://www.mesotherapyhandbook.com/patientcare-aftermesotherapy.htmlc [last accessed on 2007 Mar 15].
[Google Scholar]
|
| 25. |
Rosina P, Chieregalo C, Miccolis D, D′ Onghia FS. Psoriasis and side-effects of mesotherapy. Int J Dermatol 2001;40:581-3.
[Google Scholar]
|
| 26. |
Lee DP, Charg SE. Subcutaneous nodules showing fat necrosis owing to mesotherapy. Dermatol Srug 2005;31:250-1.
[Google Scholar]
|
| 27. |
Sañudo A, Vallejo F, Sierra M, Hoyos JG, Yepes S, Wolff JC, et al. Nontuberculous mycobacteria infection after mesotherapy: Preliminary report of 15 cases. Int J Dermatol 2007;46:649-53.
[Google Scholar]
|
| 28. |
Al-Khenaizan S. Facial cutaneous ulcers following mesotherapy. Dermatol Surg 2008;34:832-4.
[Google Scholar]
|
| 29. |
Kadry R, Hamadah I, Al-Issa A, Field L, Alrabiah F. Multifocal scalp abscess with subcutaneous fat necrosis and scarring alopecia as a complication of scalp mesotherapy. J Drugs Dermatol 2008;7:72-3.
[Google Scholar]
|
| 30. |
Urbani CE. Urticarial reaction to ethylenediamine in aminophylline following mesotherapy. Contact Derm 1994;31:198-9.
[Google Scholar]
|
| 31. |
Grojean MF, Vaillant L. Lichenoid eruption caused by mesotherapy. Ann Med Interne (Paris) 1995;146:365-6.
[Google Scholar]
|
| 32. |
Bassis D, Guilhou JJ, Giullot B. Localized urticaria pigmentosa triggered by mesotherapy. Dermatology 2004;209:343-4.
[Google Scholar]
|
| 33. |
Strahen JE, Cohen JL, Chorny JA. Granuloma annulare as a complication of mesotherapy a case report. Dermatol Surg 2008;34:836-8.
[Google Scholar]
|
| 34. |
Davies MD, Wright TI, Shehan JE. A complication of mesotherapy: Noninfectious gramulomatous panniculitis. Arch Dermatol 2008;144:808-9.
[Google Scholar]
|
| 35. |
Duquo-Estrada B, Vincenzi C, Misciali C, Tosti A. Alopecia secondary to mesotherapy. J Am Acad Dermatol 2009;61:707-9.
[Google Scholar]
|
| 36. |
Khoo AA Branford OA, Javaid M. Self injection of lipase - an extreme case for regulation in non-surgical cosmetic procedures. J Plast Reconstr Aesthet Surg 2010;63:e6-8.
[Google Scholar]
|
| 37. |
Outbreak of mesotherapy-associated skin reactions--District of Columbia area, January-February 2005. MMWR Morb Mortal Wkly Rep 2005;54:1127-30.
[Google Scholar]
|
| 38. |
Atiyeh BS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: Between scientific evidence, science fiction, and lucrative business. Aesth Plast Surg 2008:32:842-9.
[Google Scholar]
|
| 39. |
Guidelines on aesthetic practices for doctors. Updated in October 2008. Academy of Medicine, Singaore, College of Family Physicians, Singapore, and Aesthetic Practice Oversight Committee, Singapore Medical Council.
[Google Scholar]
|
| 40. |
Goh CL. The need for evidence-based aesthetic dermatology practice. J Cutan Aesthet Surg 2009;2:65-71.
[Google Scholar]
|
Fulltext Views
7,731
PDF downloads
3,682





